Chronic stress in solid tumor development: from mechanisms to interventions
- PMID: 36707854
- PMCID: PMC9883141
- DOI: 10.1186/s12929-023-00903-9
Chronic stress in solid tumor development: from mechanisms to interventions
Abstract
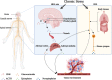
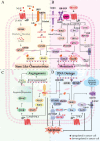
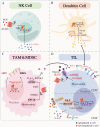
Chronic stress results in disturbances of body hormones through the neuroendocrine system. Cancer patients often experience recurrent anxiety and restlessness during disease progression and treatment, which aggravates disease progression and hinders treatment effects. Recent studies have shown that chronic stress-regulated neuroendocrine systems secret hormones to activate many signaling pathways related to tumor development in tumor cells. The activated neuroendocrine system acts not only on tumor cells but also modulates the survival and metabolic changes of surrounding non-cancerous cells. Current clinical evidences also suggest that chronic stress affects the outcome of cancer treatment. However, in clinic, there is lack of effective treatment for chronic stress in cancer patients. In this review, we discuss the main mechanisms by which chronic stress regulates the tumor microenvironment, including functional regulation of tumor cells by stress hormones (stem cell-like properties, metastasis, angiogenesis, DNA damage accumulation, and apoptotic resistance), metabolic reprogramming and immune escape, and peritumor neuromodulation. Based on the current clinical treatment framework for cancer and chronic stress, we also summarize pharmacological and non-pharmacological therapeutic approaches to provide some directions for cancer therapy.
Keywords: Cancer treatment; Chronic stress; Tumor development; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Can stress promote the pathophysiology of brain metastases? A critical review of biobehavioral mechanisms.Brain Behav Immun. 2020 Jul;87:860-880. doi: 10.1016/j.bbi.2019.12.013. Epub 2019 Dec 24. Brain Behav Immun. 2020. PMID: 31881262 Review.
-
Psychological stress on cancer progression and immunosenescence.Semin Cancer Biol. 2025 Aug;113:85-99. doi: 10.1016/j.semcancer.2025.05.007. Epub 2025 May 8. Semin Cancer Biol. 2025. PMID: 40348001 Review.
-
Harnessing Metabolic Reprogramming to Improve Cancer Immunotherapy.Int J Mol Sci. 2021 Sep 24;22(19):10268. doi: 10.3390/ijms221910268. Int J Mol Sci. 2021. PMID: 34638609 Free PMC article. Review.
-
Stem cell programs in cancer initiation, progression, and therapy resistance.Theranostics. 2020 Jul 9;10(19):8721-8743. doi: 10.7150/thno.41648. eCollection 2020. Theranostics. 2020. PMID: 32754274 Free PMC article. Review.
-
Emerging functional markers for cancer stem cell-based therapies: Understanding signaling networks for targeting metastasis.Semin Cancer Biol. 2018 Dec;53:90-109. doi: 10.1016/j.semcancer.2018.06.006. Epub 2018 Jun 30. Semin Cancer Biol. 2018. PMID: 29966677 Review.
Cited by
-
Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk.MedComm (2020). 2024 Oct 31;5(11):e784. doi: 10.1002/mco2.784. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492832 Free PMC article. Review.
-
Impact of Social Support during Diagnosis and Treatment on Disease Progression in Young Patients with Breast Cancer: A Prospective Cohort Study.Cancer Res Treat. 2024 Jan;56(1):125-133. doi: 10.4143/crt.2023.673. Epub 2023 Sep 4. Cancer Res Treat. 2024. PMID: 37669709 Free PMC article.
-
The Neuroimmune Axis and Its Therapeutic Potential for Primary Liver Cancer.Int J Mol Sci. 2024 Jun 5;25(11):6237. doi: 10.3390/ijms25116237. Int J Mol Sci. 2024. PMID: 38892423 Free PMC article. Review.
-
Genitourinary cancer and family: The reverberating psychological and cardiovascular effects of a genitourinary cancer diagnosis on first-degree relatives and spouses.Cancer. 2024 Dec 1;130(23):4061-4070. doi: 10.1002/cncr.35486. Epub 2024 Sep 9. Cancer. 2024. PMID: 39246024
-
Transcriptomic Alterations of Canine Histiocytic Sarcoma Cells in Response to Different Stressors.Int J Mol Sci. 2025 Jul 10;26(14):6629. doi: 10.3390/ijms26146629. Int J Mol Sci. 2025. PMID: 40724877 Free PMC article.
References
-
- Yuan K, Zheng YB, Wang YJ, Sun YK, Gong YM, Huang YT, Chen X, Liu XX, Zhong Y, Su SZ, et al. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: a call to action. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01638-z. - DOI - PMC - PubMed
-
- Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525–534. - PubMed
-
- Cui B, Peng F, Lu J, He B, Su Q, Luo H, Deng Z, Jiang T, Su K, Huang Y, et al. Cancer and stress: NextGen strategies. Brain Behav Immun. 2021;93:368–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81972479/National Natural Science Foundation of China
- U2004118/National Natural Science Foundation of China
- 82072899/National Natural Science Foundation of China
- 2019A1515011100/Natural Science Foundation of Guangdong Province
- 2021A1515012576/Natural Science Foundation of Guangdong Province
- 202300410359/Henan Natural Science Foundation
- SBGJ2020002081/Henan Medical Research Program
- 202005/Guangzhou High-Level Clinical Key Specialty Construction Project: Clinical Key Specialty Construction Project of Guangzhou Medical University
- 2021KTSCX026/the Innovation Project of Universities in Guangdong Province
LinkOut - more resources
Full Text Sources
Medical

