Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment
- PMID: 36707873
- PMCID: PMC9883880
- DOI: 10.1186/s40164-023-00373-7
Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment
Abstract
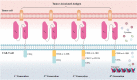
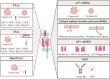
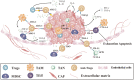
The past decade has witnessed ongoing progress in immune therapy to ameliorate human health. As an emerging technique, chimeric antigen receptor (CAR) T-cell therapy has the advantages of specific killing of cancer cells, a high remission rate of cancer-induced symptoms, rapid tumor eradication, and long-lasting tumor immunity, opening a new window for tumor treatment. However, challenges remain in CAR T-cell therapy for solid tumors due to target diversity, tumor heterogeneity, and the complex microenvironment. In this review, we have outlined the development of the CAR T-cell technique, summarized the current advances in tumor-associated antigens (TAAs), and highlighted the importance of tumor-specific antigens (TSAs) or neoantigens for solid tumors. We also addressed the challenge of the TAA binding domain in CARs to overcome off-tumor toxicity. Moreover, we illustrated the dominant tumor microenvironment (TME)-induced challenges and new strategies based on TME-associated antigens (TMAs) for solid tumor CAR T-cell therapy.
Keywords: CAR T cells; Solid tumor; TAAs; TMAs; TSAs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Liu R, Cheng Q, Kang L, Wang E, Li Y, Zhang J, et al. CD19 or CD20 CAR T cell therapy demonstrates durable antitumor efficacy in patients with central nervous system lymphoma. Hum Gene Ther. 2022;33(5–6):318–29. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

