Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: a retrospective study of a male fertility preservation cohort
- PMID: 36708005
- PMCID: PMC9977127
- DOI: 10.1093/humrep/dead004
Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: a retrospective study of a male fertility preservation cohort
Abstract
Study question: What is the impact of cancer or hematological disorders on germ cells in pediatric male patients?
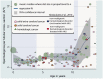
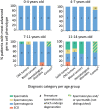
Summary answer: Spermatogonial quantity is reduced in testes of prepubertal boys diagnosed with cancer or severe hematological disorder compared to healthy controls and this reduction is disease and age dependent: patients with central nervous system cancer (CNS tumors) and hematological disorders, as well as boys <7 years are the most affected.
What is known already: Fertility preservation in pediatric male patients is considered based on the gonadotoxicity of selected treatments. Although treatment effects on germ cells have been extensively investigated, limited data are available on the effect of the disease on the prepubertal male gonad. Of the few studies investigating the effects of cancer or hematologic disorders on testicular function and germ cell quantity in prepuberty, the results are inconsistent. However, recent studies suggested impairments before the initiation of known gonadotoxic therapy. Understanding which diseases and at what age affect the germ cell pool in pediatric patients before treatment is critical to optimize strategies and counseling for fertility preservation.
Study design, size, duration: This multicenter retrospective cohort study included 101 boys aged <14 years with extra-cerebral cancer (solid tumors), CNS tumors, leukemia/lymphoma (blood cancer), or non-malignant hematological disorders, who were admitted for a fertility preservation programme between 2002 and 2018.
Participants/materials, setting, methods: In addition to clinical data, we analyzed measurements of testicular volume and performed histological staining on testicular biopsies obtained before treatment, at cryopreservation, to evaluate number of spermatogonia per tubular cross-section, tubular fertility index, and the most advanced germ cell type prior to chemo-/radiotherapy. The controls were data simulations with summary statistics from original studies reporting healthy prepubertal boys' testes characteristics.
Main results and the role of chance: Prepubertal patients with childhood cancer or hematological disorders were more likely to have significantly reduced spermatogonial quantity compared to healthy controls (48.5% versus 31.0% prevalence, respectively). The prevalence of patients with reduced spermatogonial quantity was highest in the CNS tumor (56.7%) and the hematological disorder (55.6%) groups, including patients with hydroxyurea pre-treated sickle cell disease (58.3%) and patients not exposed to hydroxyurea (50%). Disease also adversely impacted spermatogonial distribution and differentiation. Irrespective of disease, we observed the highest spermatogonial quantity reduction in patients <7 years of age.
Limitations, reasons for caution: For ethical reasons, we could not collect spermatogonial quantity data in healthy prepubertal boys as controls and thus deployed statistical simulation on data from literature. Also, our results should be interpreted considering low patient numbers per (sub)group.
Wider implications of the findings: Cancers, especially CNS tumors, and severe hematological disorders can affect spermatogonial quantity in prepubertal boys before treatment. Consequently, these patients may have a higher risk of depleted spermatogonia following therapies, resulting in persistent infertility. Therefore, patient counseling prior to disease treatment and timing of fertility preservation should not only be based on treatment regimes, but also on diagnoses and age.
Study funding/competing interest(s): This study was supported by Marie Curie Initial Training Network (ITN) (EU-FP7-PEOPLE-2013-ITN) funded by European Commision grant no. 603568; ZonMW Translational Adult stem cell research (TAS) grant no. 116003002. No competing interests.
Trial registration number: N/A.
Keywords: fertility preservation; hematological disorders; male fertility; pediatric oncology; prepubertal boys; spermatogonia; testicular tissue cryopreservation.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
Comment in
-
Male Infertility.J Urol. 2023 Nov;210(5):810-811. doi: 10.1097/JU.0000000000003672. Epub 2023 Aug 24. J Urol. 2023. PMID: 37615274 No abstract available.
Similar articles
-
Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy.Hum Reprod. 2018 Sep 1;33(9):1677-1683. doi: 10.1093/humrep/dey240. Hum Reprod. 2018. PMID: 30052981 Free PMC article.
-
Impact of low- or moderate-risk gonadotoxic chemotherapy prior to testicular tissue freezing on spermatogonia quantity in human (pre)pubertal testicular tissue.Hum Reprod. 2023 Nov 2;38(11):2105-2118. doi: 10.1093/humrep/dead161. Hum Reprod. 2023. PMID: 37674325
-
Evaluating testicular tissue for future autotransplantation: focus on cancer cell contamination and presence of spermatogonia in tissue cryobanked for boys diagnosed with a hematological malignancy.Hum Reprod. 2024 Mar 1;39(3):486-495. doi: 10.1093/humrep/dead271. Hum Reprod. 2024. PMID: 38227814
-
A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys.Hum Reprod. 2015 Nov;30(11):2463-75. doi: 10.1093/humrep/dev190. Epub 2015 Sep 10. Hum Reprod. 2015. PMID: 26358785 Review.
-
Spermatogonial stem cell preservation and transplantation: from research to clinic.Hum Reprod. 2013 Apr;28(4):897-907. doi: 10.1093/humrep/det039. Epub 2013 Feb 20. Hum Reprod. 2013. PMID: 23427228 Review.
Cited by
-
Epigenetic characterization of adult rhesus monkey spermatogonial stem cells identifies key regulators of stem cell homeostasis.Nucleic Acids Res. 2024 Dec 11;52(22):13644-13664. doi: 10.1093/nar/gkae1013. Nucleic Acids Res. 2024. PMID: 39535033 Free PMC article.
-
Optimized Recovery of Immature Germ Cells after Prepubertal Testicular Tissue Digestion and Multi-Step Differential Plating: A Step towards Fertility Restoration with Cancer-Cell-Contaminated Tissue.Int J Mol Sci. 2023 Dec 30;25(1):521. doi: 10.3390/ijms25010521. Int J Mol Sci. 2023. PMID: 38203691 Free PMC article.
-
Addressing fertility in adolescent boys with sickle cell disease: emerging clinical and ethical dilemmas.Blood Adv. 2023 Sep 26;7(18):5351-5353. doi: 10.1182/bloodadvances.2023010292. Blood Adv. 2023. PMID: 37155994 Free PMC article. No abstract available.
-
Early transcriptional states of spermatogonia and marker expressions in the prepubertal human testis following chemotherapy-induced depletion.Hum Reprod. 2025 Aug 1;40(8):1467-1475. doi: 10.1093/humrep/deaf103. Hum Reprod. 2025. PMID: 40482072 Free PMC article.
-
ESHRE good practice recommendations on fertility preservation involving testicular tissue cryopreservation in children receiving gonadotoxic therapies†.Hum Reprod. 2025 Aug 1;40(8):1391-1431. doi: 10.1093/humrep/deaf106. Hum Reprod. 2025. PMID: 40574354 Free PMC article.
References
-
- Chapman RM, Sutcliffe SB, Malpas JS.. Male gonadal dysfunction in Hodgkin’s disease: a prospective study. JAMA 1981;245:1323–1328. - PubMed
-
- Cinti S, Barbatelli G, Pierleoni C, Caucci M.. The normal, cryptorchid and retractile prepubertal human testis: a comparative morphometric ultrastructural study of 101 cases. Scanning Microsc 1993;7:351–362. - PubMed
-
- Crofton PM, Thomson AB, Evans AEM, Groome NP, Bath LE, Kelnar CJH, Wallace WHB.. Is inhibin B a potential marker of gonadotoxicity in prepubertal children treated for cancer? Clin Endocrinol (Oxf) 2003;58:296–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

