An Interventional Response Phenotyping Study in Chronic Low Back Pain: Protocol for a Mechanistic Randomized Controlled Trial
- PMID: 36708026
- PMCID: PMC10403311
- DOI: 10.1093/pm/pnad005
An Interventional Response Phenotyping Study in Chronic Low Back Pain: Protocol for a Mechanistic Randomized Controlled Trial
Abstract
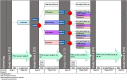
Evidence-based treatments for chronic low back pain (cLBP) typically work well in only a fraction of patients, and at present there is little guidance regarding what treatment should be used in which patients. Our central hypothesis is that an interventional response phenotyping study can identify individuals with different underlying mechanisms for their pain who thus respond differentially to evidence-based treatments for cLBP. Thus, we will conduct a randomized controlled Sequential, Multiple Assessment, Randomized Trial (SMART) design study in cLBP with the following three aims. Aim 1: Perform an interventional response phenotyping study in a cohort of cLBP patients (n = 400), who will receive a sequence of interventions known to be effective in cLBP. For 4 weeks, all cLBP participants will receive a web-based pain self-management program as part of a run-in period, then individuals who report no or minimal improvement will be randomized to: a) mindfulness-based stress reduction, b) physical therapy and exercise, c) acupressure self-management, and d) duloxetine. After 8 weeks, individuals who remain symptomatic will be re-randomized to a different treatment for an additional 8 weeks. Using those data, we will identify the subsets of participants that respond to each treatment. In Aim 2, we will show that currently available, clinically derived measures, can predict differential responsiveness to the treatments. In Aim 3, a subset of participants will receive deeper phenotyping (n = 160), to identify new experimental measures that predict differential responsiveness to the treatments, as well as to infer mechanisms of action. Deep phenotyping will include functional neuroimaging, quantitative sensory testing, measures of inflammation, and measures of autonomic tone.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Academy of Pain Medicine.
Figures
References
-
- Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA.. Epidemiology of low back pain in adults. Neuromodulation. 2014;17(Suppl 2):3–10. - PubMed
-
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. - PubMed
-
- Nuesch E, Hauser W, Bernardy K, Barth J, Juni P.. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann Rheum Dis. 2013;72(6):955–962. - PubMed
-
- Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ.. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical