Validation of a multiple marker test for early pregnancy outcome prediction
- PMID: 36708430
- PMCID: PMC10224881
- DOI: 10.1007/s10815-023-02719-w
Validation of a multiple marker test for early pregnancy outcome prediction
Abstract
Purpose: To validate the use of a multiple biomarker test panel for predicting first trimester pregnancy outcome in a multi-center cohort.
Methods: A case-control study of women presenting with pain and bleeding in early pregnancy at 5-10 weeks gestational age was performed at three academic centers. Sera from women with ectopic pregnancy (EP), viable intrauterine pregnancy (IUP), and miscarriage (SAB) were analyzed via immunoassay for Activin A (AA), Progesterone (P4), A Disintegrin And Metalloprotease-12 (ADAM12), pregnancy-associated plasma protein A (PAPP-A), glycodelin (Glyc), and human chorionic gonadotropin (hCG). Biomarkers were assessed for reproducibility using medians, ranges, standard deviations, and area under receiver-operating characteristic curve (AUC) and accuracy in early pregnancy outcome classification compared to a previous derivation population.
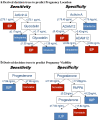
Results: In 192 pregnancies, the biomarkers demonstrated good reproducibility with similar medians, ranges, and AUCs when compared to the derivation population except glycodelin. Pregnancy location was conclusively classified in 53% (n = 94) of the whole study sample with 78% accuracy. Pregnancy viability was conclusively classified in 58% (n = 112) of the new sample with 89% accuracy. Results were similar with subsequent model revisions where glycodelin was excluded and in the subgroups of subjects with a hCG below 2000 mIU/mL and a gestational age less than 6 weeks.
Conclusion: The use of a panel of biomarkers to maximize test accuracy of a prediction of pregnancy location and prediction of pregnancy viability was reproducible and validated in an external population from which it was derived, but clinical utility is limited based on the test characteristics obtained.
Keywords: Biomarker; Ectopic pregnancy; Validation.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
First trimester ultrasound tests alone or in combination with first trimester serum tests for Down's syndrome screening.Cochrane Database Syst Rev. 2017 Mar 15;3(3):CD012600. doi: 10.1002/14651858.CD012600. Cochrane Database Syst Rev. 2017. PMID: 28295158 Free PMC article.
-
First trimester serum tests for Down's syndrome screening.Cochrane Database Syst Rev. 2015 Nov 30;2015(11):CD011975. doi: 10.1002/14651858.CD011975. Cochrane Database Syst Rev. 2015. PMID: 26617074 Free PMC article.
-
Intrauterine administration of human chorionic gonadotropin (hCG) for subfertile women undergoing assisted reproduction.Cochrane Database Syst Rev. 2016 May 20;(5):CD011537. doi: 10.1002/14651858.CD011537.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Oct 20;10:CD011537. doi: 10.1002/14651858.CD011537.pub3. PMID: 27195724 Updated.
-
Multiplexed serum biomarkers to discriminate nonviable and ectopic pregnancy.Fertil Steril. 2024 Sep;122(3):482-493. doi: 10.1016/j.fertnstert.2024.04.028. Epub 2024 Apr 26. Fertil Steril. 2024. PMID: 38677710
-
Predicting first trimester pregnancy outcome: derivation of a multiple marker test.Fertil Steril. 2016 Dec;106(7):1725-1732.e3. doi: 10.1016/j.fertnstert.2016.08.044. Epub 2016 Oct 26. Fertil Steril. 2016. PMID: 28340932 Free PMC article.
Cited by
-
Liability, risks, and recommendations for ultrasound use in the diagnosis of obstetrics diseases.Heliyon. 2023 Nov 4;9(11):e21829. doi: 10.1016/j.heliyon.2023.e21829. eCollection 2023 Nov. Heliyon. 2023. Retraction in: Heliyon. 2025 Apr 15;11(9):e43333. doi: 10.1016/j.heliyon.2025.e43333. PMID: 38045126 Free PMC article. Retracted. Review.
-
PAPPA's role in female reproduction.Reproduction. 2025 Jul 18;170(2):e250012. doi: 10.1530/REP-25-0012. Print 2025 Aug 1. Reproduction. 2025. PMID: 40683301 Free PMC article. Review.
-
Designing an Electrochemical Biosensor Based on Voltammetry for Measurement of Human Chorionic Gonadotropin.J Med Signals Sens. 2024 Jul 25;14:21. doi: 10.4103/jmss.jmss_64_23. eCollection 2024. J Med Signals Sens. 2024. PMID: 39234593 Free PMC article.
References
-
- Barnhart KT, Simhan H, Kamelle SA. Diagnostic accuracy of ultrasound above and below the beta-hCG discriminatory zone. Obstetrics & Gynecology. 1999;94:583–587. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

