Regulation of beige adipocyte thermogenesis by the cold-repressed ER protein NNAT
- PMID: 36708951
- PMCID: PMC9932177
- DOI: 10.1016/j.molmet.2023.101679
Regulation of beige adipocyte thermogenesis by the cold-repressed ER protein NNAT
Abstract
Objective: Cold stimuli trigger the conversion of white adipose tissue into beige adipose tissue, which is capable of non-shivering thermogenesis. However, what process drives this activation of thermogenesis in beige fat is not well understood. Here, we examine the ER protein NNAT as a regulator of thermogenesis in adipose tissue.
Methods: We investigated the regulation of adipose tissue NNAT expression in response to changes in ambient temperature. We also evaluated the functional role of NNAT in thermogenic regulation using Nnat null mice and primary adipocytes that lack or overexpress NNAT.
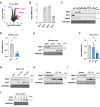
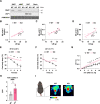
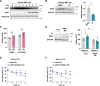
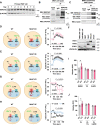
Results: Cold exposure or treatment with a β3-adrenergic agonist reduces the expression of adipose tissue NNAT in mice. Genetic disruption of Nnat in mice enhances inguinal adipose tissue thermogenesis. Nnat null mice exhibit improved cold tolerance both in the presence and absence of UCP1. Gain-of-function studies indicate that ectopic expression of Nnat abolishes adrenergic receptor-mediated respiration in beige adipocytes. NNAT physically interacts with the ER Ca2+-ATPase (SERCA) in adipocytes and inhibits its activity, impairing Ca2+ transport and heat dissipation. We further demonstrate that NHLRC1, an E3 ubiquitin protein ligase implicated in proteasomal degradation of NNAT, is induced by cold exposure or β3-adrenergic stimulation, thus providing regulatory control at the protein level. This serves to link cold stimuli to NNAT degradation in adipose tissue, which in turn leads to enhanced SERCA activity.
Conclusions: Our study implicates NNAT in the regulation of adipocyte thermogenesis.
Keywords: Beige fat; NHLRC1; NNAT; SERCA2; Thermogenesis.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- van der Klaauw A.A., Farooqi I.S. The hunger genes: pathways to obesity. Cell. 2015;161:119–132. - PubMed
-
- Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. - PubMed
-
- Chouchani E.T., Kazak L., Spiegelman B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metabol. 2019;29:27–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

