Agmatine reduces alcohol drinking and produces antinociceptive effects in rodent models of alcohol use disorder
- PMID: 36709008
- PMCID: PMC10175169
- DOI: 10.1016/j.alcohol.2023.01.003
Agmatine reduces alcohol drinking and produces antinociceptive effects in rodent models of alcohol use disorder
Abstract
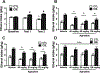
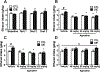
Alcohol use disorder (AUD) is a chronic, relapsing disorder characterized by an escalation of drinking and the emergence of negative affective states over time. Within this framework, alcohol may be used in excessive amounts to alleviate withdrawal-related symptoms, such as hyperalgesia. Future effective therapeutics for AUD may need to exhibit the ability to reduce drinking as well as to alleviate co-morbid conditions such as pain, and to take mechanistic sex differences into consideration. Agmatine is an endogenous neuromodulator that has been previously implicated in the regulation of reward and pain processing. In the current set of studies, we examined the ability of agmatine to reduce escalated ethanol drinking in complementary models of AUD where adult male and female mice and rats were made dependent via chronic, intermittent ethanol vapor exposure (CIE). We also examined the ability of agmatine to modify thermal and mechanical sensitivity in alcohol-dependent male and female rats. Agmatine reduced alcohol drinking in a dose-dependent fashion, with somewhat greater selectivity in alcohol-dependent female mice (versus non-dependent female mice), but equivalent efficacy across male mice and both groups of male and female rats. In mice and female rats, this efficacy did not extend to sucrose drinking, indicating some selectivity for ethanol reinforcement. Female rats made dependent on alcohol demonstrated significant hyperalgesia symptoms, and agmatine produced dose-dependent antinociceptive effects across both sexes. While additional mechanistic studies into agmatine are necessary, these findings support the broad-based efficacy of agmatine to treat co-morbid excessive drinking and pain symptoms in the context of AUD.
Keywords: alcohol dependence; analgesia; ethanol; pain; self-administration.
Published by Elsevier Inc.
Conflict of interest statement
Author Disclosure Statement
The authors declare no competing financial interests or potential conflicts of interest.
Figures
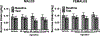
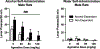
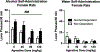
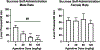
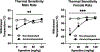
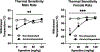
Similar articles
-
Kappa-opioid receptor antagonism in the nucleus accumbens shell distinguishes escalated alcohol consumption and negative affective-like behavior from physiological withdrawal in alcohol-dependence.Pharmacol Biochem Behav. 2024 Oct;243:173840. doi: 10.1016/j.pbb.2024.173840. Epub 2024 Aug 7. Pharmacol Biochem Behav. 2024. PMID: 39096973
-
Exploring Sex Differences in the Attenuation of Ethanol Drinking by Naltrexone in Dependent Rats During Early and Protracted Abstinence.Alcohol Clin Exp Res. 2018 Dec;42(12):2466-2478. doi: 10.1111/acer.13898. Epub 2018 Oct 28. Alcohol Clin Exp Res. 2018. PMID: 30320880 Free PMC article.
-
Analgesic effect of oxytocin in alcohol-dependent male and female rats.Alcohol. 2025 Mar;123:27-38. doi: 10.1016/j.alcohol.2024.12.002. Epub 2024 Dec 21. Alcohol. 2025. PMID: 39716604
-
Alcohol dependence and free-choice drinking in mice.Alcohol. 2014 May;48(3):287-93. doi: 10.1016/j.alcohol.2013.11.006. Epub 2014 Jan 18. Alcohol. 2014. PMID: 24530006 Free PMC article. Review.
-
Sex Differences in Mouse Models of Voluntary Alcohol Drinking and Abstinence-Induced Negative Emotion.Alcohol. 2024 Dec;121:45-57. doi: 10.1016/j.alcohol.2024.07.004. Epub 2024 Jul 23. Alcohol. 2024. PMID: 39053705 Review.
Cited by
-
Within and Beyond the Binary: Sex and Gender Differences in Pain and Alcohol Use Disorder.Curr Addict Rep. 2024 Feb;11(1):68-80. doi: 10.1007/s40429-023-00534-y. Epub 2023 Dec 22. Curr Addict Rep. 2024. PMID: 40747361 Free PMC article.
-
Targeting IL-6 as a novel therapeutic approach for alcohol abstinence - related mechanical allodynia.Neuropharmacology. 2025 Nov 1;278:110584. doi: 10.1016/j.neuropharm.2025.110584. Epub 2025 Jun 28. Neuropharmacology. 2025. PMID: 40588184
-
Increased alcohol-biased choice behavior in mouse models of high alcohol drinking.Alcohol Clin Exp Res (Hoboken). 2025 Jul;49(7):1435-1444. doi: 10.1111/acer.70076. Epub 2025 May 22. Alcohol Clin Exp Res (Hoboken). 2025. PMID: 40405336 Free PMC article.
-
Chronic intermittent ethanol produces nociception through endocannabinoid-independent mechanisms in mice.bioRxiv [Preprint]. 2025 Feb 4:2024.11.08.622656. doi: 10.1101/2024.11.08.622656. bioRxiv. 2025. Update in: Neuropharmacology. 2025 Oct 1;277:110502. doi: 10.1016/j.neuropharm.2025.110502. PMID: 39975399 Free PMC article. Updated. Preprint.
-
Histone deacetylase inhibitor decreases hyperalgesia in a mouse model of alcohol withdrawal-induced hyperalgesia.Alcohol Clin Exp Res (Hoboken). 2024 Mar;48(3):478-487. doi: 10.1111/acer.15273. Epub 2024 Feb 20. Alcohol Clin Exp Res (Hoboken). 2024. PMID: 38378262 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

