The classical pathway triggers pathogenic complement activation in membranous nephropathy
- PMID: 36709213
- PMCID: PMC9884226
- DOI: 10.1038/s41467-023-36068-0
The classical pathway triggers pathogenic complement activation in membranous nephropathy
Abstract
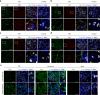
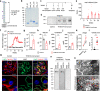
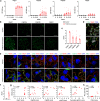
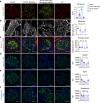
Membranous nephropathy (MN) is an antibody-mediated autoimmune disease characterized by glomerular immune complexes containing complement components. However, both the initiation pathways and the pathogenic significance of complement activation in MN are poorly understood. Here, we show that components from all three complement pathways (alternative, classical and lectin) are found in renal biopsies from patients with MN. Proximity ligation assays to directly visualize complement assembly in the tissue reveal dominant activation via the classical pathway, with a close correlation to the degree of glomerular C1q-binding IgG subclasses. In an antigen-specific autoimmune mouse model of MN, glomerular damage and proteinuria are reduced in complement-deficient mice compared with wild-type littermates. Severe disease with progressive ascites, accompanied by extensive loss of the integral podocyte slit diaphragm proteins, nephrin and neph1, only occur in wild-type animals. Finally, targeted silencing of C3 using RNA interference after the onset of proteinuria significantly attenuates disease. Our study shows that, in MN, complement is primarily activated via the classical pathway and targeting complement components such as C3 may represent a promising therapeutic strategy.
© 2023. The Author(s).
Conflict of interest statement
A.B. is an employee and shareholder of Alnylam Pharmaceuticals. P.F.Z. serves as a consultant for Eleva GMBH, Novartis, Generic Assays, Alexion, Bayer, Vifor Fresenius Medical Care Renal Pharma, and Samsung Bioepis. All other authors declare no competing interests. P.F., S.W., and T.W. applied for a patent “C3/C5 convertase assays” (EP 3771468/US-2022-0276261).
Figures


References
-
- Maisonneuve P, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am. J. Kidney Dis. 2000;35:157–165. - PubMed
-
- Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology: membranous nephropathy. Am. J. Kidney Dis. 2015;66:e15–e17. - PubMed
-
- Segawa Y, et al. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr. Nephrol. 2010;25:1091–1099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

