A complex ePrescribing-based Anti-Microbial Stewardship (ePAMS+) intervention for hospitals combining technological and behavioural components: protocol for a feasibility trial
- PMID: 36709308
- PMCID: PMC9883604
- DOI: 10.1186/s40814-022-01230-w
A complex ePrescribing-based Anti-Microbial Stewardship (ePAMS+) intervention for hospitals combining technological and behavioural components: protocol for a feasibility trial
Abstract
Background: Antimicrobial resistance is a leading global public health threat, with inappropriate use of antimicrobials in healthcare contributing to its development. Given this urgent need, we developed a complex ePrescribing-based Anti-Microbial Stewardship intervention (ePAMS+).
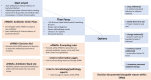
Methods: ePAMS+ includes educational and organisational behavioural elements, plus guideline-based clinical decision support to aid optimal antimicrobial use in hospital inpatients. ePAMS+ particularly focuses on prompt initiation of antimicrobials, followed by early review once test results are available to facilitate informed decision-making on stopping or switching where appropriate. A mixed-methods feasibility trial of ePAMS+ will take place in two NHS acute hospital care organisations. Qualitative staff interviews and observation of practice will respectively gather staff views on the technical component of ePAMS+ and information on their use of ePAMS+ in routine work. Focus groups will elicit staff and patient views on ePAMS+; one-to-one interviews will discuss antimicrobial stewardship with staff and will record patient experiences of receiving antibiotics and their thoughts on inappropriate prescribing. Qualitative data will be analysed thematically. Fidelity Index development will enable enactment of ePAMS+ to be measured objectively in a subsequent trial assessing the effectiveness of ePAMS+. Quantitative data collection will determine the feasibility of extracting data and deriving key summaries of antimicrobial prescribing; we will quantify variability in the primary outcome, number of antibiotic defined daily doses, to inform the future larger-scale trial design.
Discussion: This trial is essential to determine the feasibility of implementing the ePAMS+ intervention and measuring relevant outcomes, prior to evaluating its clinical and cost-effectiveness in a full scale hybrid cluster-randomised stepped-wedge clinical trial. Findings will be shared with study sites and with qualitative research participants and will be published in peer-reviewed journals and presented at academic conferences.
Trial registration: The qualitative and Fidelity Index research were approved by the Health and Research Authority and the North of Scotland Research Ethics Service (ref: 19/NS/0174). The feasibility trial and quantitative analysis (protocol v1.0, 15 December 2021) were approved by the London South East Research Ethics Committee (ref: 22/LO/0204) and registered with ISRCTN ( ISRCTN 13429325 ) on 24 March 2022.
Keywords: Bacteriology; Decision support; Health informatics; Infectious diseases; Microbiology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
A complex ePrescribing antimicrobial stewardship-based (ePAMS+) intervention for hospitals: mixed-methods feasibility trial results.BMC Med Inform Decis Mak. 2024 Oct 11;24(1):301. doi: 10.1186/s12911-024-02707-9. BMC Med Inform Decis Mak. 2024. PMID: 39394550 Free PMC article.
-
Antibiotic Review Kit for Hospitals (ARK-Hospital): study protocol for a stepped-wedge cluster-randomised controlled trial.Trials. 2019 Jul 11;20(1):421. doi: 10.1186/s13063-019-3497-y. Trials. 2019. PMID: 31296255 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Cue-based versus scheduled feeding for preterm infants transitioning from tube to oral feeding: the Cubs mixed-methods feasibility study.Health Technol Assess. 2021 Dec;25(74):1-146. doi: 10.3310/hta25740. Health Technol Assess. 2021. PMID: 34878383
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
Cited by
-
Complex Hospital-Based Electronic Prescribing-Based Intervention to Support Antimicrobial Stewardship: Qualitative Study.JMIR Form Res. 2024 Jul 26;8:e54458. doi: 10.2196/54458. JMIR Form Res. 2024. PMID: 39059001 Free PMC article.
-
A complex ePrescribing antimicrobial stewardship-based (ePAMS+) intervention for hospitals: mixed-methods feasibility trial results.BMC Med Inform Decis Mak. 2024 Oct 11;24(1):301. doi: 10.1186/s12911-024-02707-9. BMC Med Inform Decis Mak. 2024. PMID: 39394550 Free PMC article.
References
-
- World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. Geneva: World Health Organization; 2021.
-
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Geneva: World Health Organization; 2015. - PubMed
-
- O’Neill J. Review on antimicrobial resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; 2014.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

