A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens
- PMID: 36709333
- PMCID: PMC9883832
- DOI: 10.1038/s41467-023-36101-2
A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens
Abstract
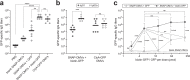
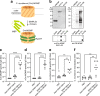
Engineered outer membrane vesicles (OMVs) derived from Gram-negative bacteria are a promising technology for the creation of non-infectious, nanoparticle vaccines against diverse pathogens. However, antigen display on OMVs can be difficult to control and highly variable due to bottlenecks in protein expression and localization to the outer membrane of the host cell, especially for bulky and/or complex antigens. Here, we describe a universal approach for avidin-based vaccine antigen crosslinking (AvidVax) whereby biotinylated antigens are linked to the exterior of OMVs whose surfaces are remodeled with multiple copies of a synthetic antigen-binding protein (SNAP) comprised of an outer membrane scaffold protein fused to a biotin-binding protein. We show that SNAP-OMVs can be readily decorated with a molecularly diverse array of biotinylated subunit antigens, including globular and membrane proteins, glycans and glycoconjugates, haptens, lipids, and short peptides. When the resulting OMV formulations are injected in mice, strong antigen-specific antibody responses are observed that depend on the physical coupling between the antigen and SNAP-OMV delivery vehicle. Overall, these results demonstrate AvidVax as a modular platform that enables rapid and simplified assembly of antigen-studded OMVs for application as vaccines against pathogenic threats.
© 2023. The Author(s).
Conflict of interest statement
M.P.D. has a financial interest in Gauntlet, Inc., Glycobia, Inc., MacImmune, Inc., SwiftScale, Inc., and UbiquiTX, Inc. M.P.D. and D.P. have a financial interest in Versatope Therapeutics, Inc. M.P.D.’s and D.P.’s interests are reviewed and managed by Cornell University in accordance with their conflict-of-interest policies. M.P.D. has no non-financial competing interests to declare. All other authors declare no competing interests.
Figures



References
-
- Biller SJ, et al. Bacterial vesicles in marine ecosystems. Science. 2014;343:183–186. - PubMed

