Metamorphism in TDP-43 prion-like domain determines chaperone recognition
- PMID: 36709343
- PMCID: PMC9884275
- DOI: 10.1038/s41467-023-36023-z
Metamorphism in TDP-43 prion-like domain determines chaperone recognition
Abstract
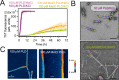
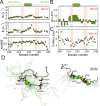
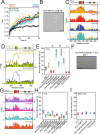
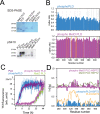
The RNA binding protein TDP-43 forms cytoplasmic inclusions via its C-terminal prion-like domain in several neurodegenerative diseases. Aberrant TDP-43 aggregation arises upon phase de-mixing and transitions from liquid to solid states, following still unknown structural conversions which are primed by oxidative stress and chaperone inhibition. Despite the well-established protective roles for molecular chaperones against protein aggregation pathologies, knowledge on the determinants of chaperone recognition in disease-related prions is scarce. Here we show that chaperones and co-chaperones primarily recognize the structured elements in TDP-43´s prion-like domain. Significantly, while HSP70 and HSP90 chaperones promote TDP-43 phase separation, co-chaperones from the three classes of the large human HSP40 family (namely DNAJA2, DNAJB1, DNAJB4 and DNAJC7) show strikingly different effects on TDP-43 de-mixing. Dismantling of the second helical element in TDP-43 prion-like domain by methionine sulfoxidation impacts phase separation and amyloid formation, abrogates chaperone recognition and alters phosphorylation by casein kinase-1δ. Our results show that metamorphism in the post-translationally modified TDP-43 prion-like domain encodes determinants that command mechanisms with major relevance in disease.
© 2023. The Author(s).
Conflict of interest statement
J.C., R.A., D.P.U., D.V.L., M.G., and J.O. are co-inventors of the patent application
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

