Neuroligin-2 controls the establishment of fast GABAergic transmission in adult-born granule cells
- PMID: 36709408
- PMCID: PMC11342305
- DOI: 10.1002/hipo.23505
Neuroligin-2 controls the establishment of fast GABAergic transmission in adult-born granule cells
Abstract
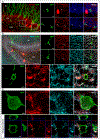
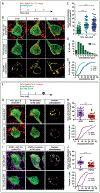
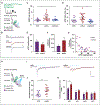
GABAergic inhibition is critical for the precision of neuronal spiking and the homeostatic regulation of network activity in the brain. Adult neurogenesis challenges network homeostasis because new granule cells (GCs) integrate continuously in the functional dentate gyrus. While developing, adult-born GCs undergo a transient state of enhanced excitability due to the delayed maturation of perisomatic GABAergic inhibition by parvalbumin interneurons (PV-INs). The mechanisms underlying this delayed synaptic maturation remain unknown. We examined the morphology and function of synapses formed by PV-INs onto new GCs over a 2-month interval in young adult mice, and investigated the influence of the synaptic adhesion molecule neuroligin-2 (NL2). Perisomatic appositions of PV-IN terminals onto new GCs were conspicuous at 2 weeks and continued to grow in size to reach a plateau over the fourth week. Postsynaptic knockdown of NL2 by expression of a short-hairpin RNA (shNL2) in new GCs resulted in smaller size of synaptic contacts, reduced area of perisomatic appositions of the vesicular GABA transporter VGAT, and the number of presynaptic active sites. GCs expressing shNL2 displayed spontaneous GABAergic responses with decreased frequency and amplitude, as well as slower kinetics compared to control GCs. In addition, postsynaptic responses evoked by optogenetic stimulation of PV-INs exhibited slow kinetics, increased paired-pulse ratio and coefficient of variation in GCs with NL2 knockdown, suggesting a reduction in the number of active synapses as well as in the probability of neurotransmitter release (Pr ). Our results demonstrate that synapses formed by PV-INs on adult-born GCs continue to develop beyond the point of anatomical growth, and require NL2 for the structural and functional maturation that accompanies the conversion into fast GABAergic transmission.
Keywords: adult neurogenesis; dentate gyrus; excitability; interneurons; neural development; plasticity; synaptogenesis.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

