Rates of regional tau accumulation in ageing and across the Alzheimer's disease continuum: an AIBL 18F-MK6240 PET study
- PMID: 36709581
- PMCID: PMC9900352
- DOI: 10.1016/j.ebiom.2023.104450
Rates of regional tau accumulation in ageing and across the Alzheimer's disease continuum: an AIBL 18F-MK6240 PET study
Abstract
Background: Tau positron emission tomography (PET) imaging enables longitudinal observation of tau accumulation in Alzheimer's disease (AD). 18F-MK6240 is a high affinity tracer for the paired helical filaments of tau in AD, widely used in clinical trials, despite sparse longitudinal natural history data. We aimed to evaluate the natural history of tau accumulation, and the impact of disease stage and reference region on the magnitude and effect size of regional change.
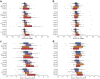
Methods: One hundred and eighty-four participants: 89 cognitively unimpaired (CU) beta-amyloid negative (Aβ-), 44 CU Aβ+, 51 cognitively impaired Aβ+ (26 with mild cognitive impairment [MCI] and 25 with dementia) had follow-up 18F-MK6240 PET for one to four years (median 1.48). Regional standardised uptake value ratios (SUVR) were generated. Two reference regions were examined: cerebellar cortex and eroded subcortical white matter.
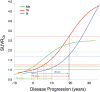
Findings: CU Aβ- participants had very low rates of tau accumulation in the mesial temporal lobe (MTL). In CU Aβ+, significantly higher rate of accumulation was seen in the MTL (particularly the amygdala), extending into the inferior temporal lobes. In MCI Aβ+, the rate of accumulation was greatest in the lateral temporal, parietal and lateral occipital cortex, and plateaued in the MTL. Accumulation was global in AD Aβ+, except for a plateau in the MTL. The eroded subcortical white matter reference region showed no significant advantage over the cerebellar cortex and appeared prone to spill-over in AD participants. Data fitting suggested approximately 15-20 years to accumulate tau to typical AD levels.
Interpretation: Tau accumulation occurs slowly. Rates vary according to brain region, disease stage and tend to plateau at high levels. Rates of tau accumulation are best measured in the MTL and inferior temporal cortex in preclinical AD and in large neocortical areas, in MCI and AD.
Funding: NHMRC; Cerveau Technologies.
Keywords: (18)F-MK6240; Alzheimer's disease; Longitudinal; Positron emission tomography (PET); Tau.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests CCR was the recipient of a research grant from Cerveau (institution), who supplied the MK6240 tau tracer precursor for research use. CCR has received consulting fees from Prothera and Merck (scientific advisory panels) and Biogen (for preparation of educational material). CCR has received support for attending meetings and/or travel from Cerveau and the Alzheimer's Association. VLV has received consulting fees from IXICO, Eli Lilly, Life molecular imaging and has received payment/honoraria from ACE Barcelona. VLV has participated on the data safety monitoring/advisory board of Eli Lilly. JF was the recipient of a research grant from the National Health and Medical Research Council (NHMRC), grant numbers APP1132604 and APP1140955. NK, VD, JR, LW, CF, PB, and CLM do not report any disclosures.
Figures

References
-
- Devous M.D., Fleisher A.S., Pontecorvo M.J., et al. Relationships between cognition and neuropathological tau in alzheimer's disease assessed by 18F flortaucipir PET. J Alzheimers Dis. 2021;80(3):1091–1104. - PubMed
-
- Mattsson N., Insel P.S., Donohue M., et al. Predicting diagnosis and cognition with 18F-AV-1451 tau PET and structural MRI in Alzheimer's disease. Alzheimers Dement. 2019;15(4):570–580. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

