ADAR1 and ZBP1 in innate immunity, cell death, and disease
- PMID: 36710220
- PMCID: PMC9974732
- DOI: 10.1016/j.it.2023.01.001
ADAR1 and ZBP1 in innate immunity, cell death, and disease
Abstract
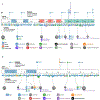
ADAR1 and ZBP1 are the only two mammalian proteins that contain Zα domains, which are thought to bind to nucleic acids in the Z-conformation. These two molecules are crucial in regulating diverse biological processes. While ADAR1-mediated RNA editing supports host survival and development, ZBP1-mediated immune responses provide host defense against infection and disease. Recent studies have expanded our understanding of the functions of ADAR1 and ZBP1 beyond their classical roles and established their fundamental regulation of innate immune responses, including NLRP3 inflammasome activation, inflammation, and cell death. Their roles in these processes have physiological impacts across development, infectious and inflammatory diseases, and cancer. In this review, we discuss the functions of ADAR1 and ZBP1 in regulating innate immune responses in development and disease.
Keywords: ADAR1; NLRP3; PANoptosis; PANoptosome; RIPK1; RIPK3; ZBP1; Zα domain; apoptosis; cancer; caspase; cell death; development; homeostasis; infection; inflammasome; inflammation; innate immunity; necroptosis; pyroptosis; tumorigenesis.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests T.-D.K. is a consultant for Pfizer.
Figures
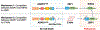

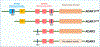

References
-
- Wu J and Chen ZJ (2014) Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol 32, 461–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

