RNAi-based knockdown of candidate gut receptor genes altered the susceptibility of Spodoptera frugiperda and S. litura larvae to a chimeric toxin Cry1AcF
- PMID: 36710863
- PMCID: PMC9881468
- DOI: 10.7717/peerj.14716
RNAi-based knockdown of candidate gut receptor genes altered the susceptibility of Spodoptera frugiperda and S. litura larvae to a chimeric toxin Cry1AcF
Abstract
Background: A multitude of Cry toxins (secreted by Bacillus thuringiensis or Bt) has been deployed globally either via transgenic mean or bio-pesticidal formulations in order to manage insect pests. However, Bt resistance development in insects is emerging as a major concern. To avoid this problem, multiple gene pyramiding or protein-engineered chimeric toxin-based strategy has been analyzed.
Methods: In the present study, one such chimeric toxin Cry1AcF (contain the swapped domains of Cry1Ac and Cry1F) was used to investigate its in vivo pathogenesis process in lepidopteran pests Spodoptera frugiperda and S. litura. A number of biochemical and molecular analysis were performed.
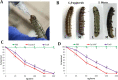
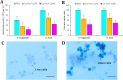
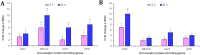
Results: Oral ingestion of Cry1AcF caused greater toxicity in S. frugiperda than S. litura with larvae displaying increased hemolymph melanization. Histopathology of the midgut transverse sections exhibited Cry1AcF-induced extensive gut damage in both the test insects followed by cytotoxicity in terms of reduced hemocyte numbers and viability. Elevated hemolymph phenoloxidase activity indicated the immune-stimulatory nature of Cry1AcF. In order to analyze the role of gut receptor proteins in Cry1AcF intoxication in test insects, we performed RNAi-mediated silencing using bacterially-expressed dsRNAs of individual receptor-encoding genes including CAD, ABCC2, ALP1 and APN. Target-specific induced downregulation of receptor mRNAs differentially altered the insect susceptibility to Cry1AcF toxin in our study. The susceptibility of ALP1 and APN dsRNA pre-treated S. frugiperda was considerably decreased when treated with Cry1AcF in LD50 and LD90 doses, whereas susceptibility of CAD and ABCC2 dsRNA pre-treated S. litura was significantly reduced when ingested with Cry1AcF in different doses. CAD/ABCC2-silenced S. frugiperda and ALP1/APN-silenced S. litura were vulnerable to Cry1AcF alike of control larvae. In conclusion, our results indicate ALP1/APN and CAD/ABCC2 as the functional receptor for Cry1AcF toxicity in S. frugiperda and S. litura, respectively.
Keywords: Cry receptor-encoding genes; Gene silencing; Hemocyte viability; Histopathology; Insect mortality; PO activity.
© 2023 Dutta et al.
Conflict of interest statement
Tushar K. Dutta is an Academic Editor for PeerJ.
Figures


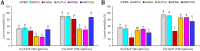
References
-
- Adang MJ, Crickmore N, Jurat-Fuentes JL. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In: Dhadialla TS, Gill SS, editors. Advances in Insect Physiology: Insect Midgut and Insecticidal Proteins. Vol. 47. San Francisco: Elsevier; 2014. pp. 39–87.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

