In Silico Design and In Vitro Evaluation of Some Novel AMPs Derived From Human LL-37 as Potential Antimicrobial Agents for Keratitis
- PMID: 36710989
- PMCID: PMC9872548
- DOI: 10.5812/ijpr-124017
In Silico Design and In Vitro Evaluation of Some Novel AMPs Derived From Human LL-37 as Potential Antimicrobial Agents for Keratitis
Abstract
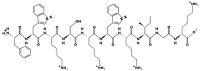
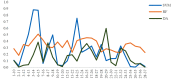
The human body produces two classes of antimicrobial peptides (AMPs), namely defensins and cathelicidins. In this study, a novel decapeptide (Catoid) and its dimer (Dicatoid) based on human cathelicidin (LL-37) have been designed by bioinformatics tools to be used in the treatment of bacterial keratitis. After the selection and synthesis of peptide sequences, their antimicrobial activities against the standard and resistant strains of Pseudomonas aeruginosa and Staphylococcus aureus were evaluated. This test was performed with LL-37, gentamicin, ciprofloxacin, amikacin, and penicillin for a more accurate comparison. Furthermore, the cytotoxicity levels of the specified compounds on fibroblast cells and bovine corneal endothelial cells were investigated. The results demonstrated that the designed peptides had a superior antimicrobial activity on P. aeruginosa, compared to LL-37; however, Catoid had a better effect on the S. aureus strain. Additionally, a significant achievement is the very low toxicity level of Catoid and Dicatoid on the human skin fibroblast cell line and bovine corneal endothelial cells, compared to that of LL-37 as the initial design model.
Keywords: Antimicrobial Peptides (AMPs); Bacterial Keratitis; Bovine Corneal Endothelial Cells; Cathelicidin; Catoid; Dicatoid.
Copyright © 2022, Author(s).
Conflict of interest statement
Conflict of Interests: The authors have no conflict of interest to declare.
Figures



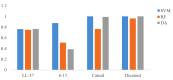



References
-
- Ting DSJ, Ho CS, Cairns J, Elsahn A, Al-Aqaba M, Boswell T, et al. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham Infectious Keratitis Study. Br J Ophthalmol. 2021;105(3):328–33. doi: 10.1136/bjophthalmol-2020-316128. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
