Facile Functionalization of Carbon Electrodes for Efficient Electroenzymatic Hydrogen Production
- PMID: 36711103
- PMCID: PMC9875370
- DOI: 10.1021/jacsau.2c00551
Facile Functionalization of Carbon Electrodes for Efficient Electroenzymatic Hydrogen Production
Abstract
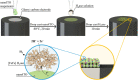
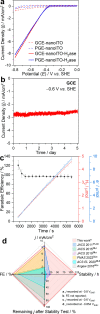
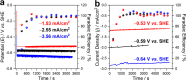
Enzymatic electrocatalysis holds promise for new biotechnological approaches to produce chemical commodities such as molecular hydrogen (H2). However, typical inhibitory limitations include low stability and/or low electrocatalytic currents (low product yields). Here we report a facile single-step electrode preparation procedure using indium-tin oxide nanoparticles on carbon electrodes. The subsequent immobilization of a model [FeFe]-hydrogenase from Clostridium pasteurianum ("CpI") on the functionalized carbon electrode permits comparatively large quantities of H2 to be produced in a stable manner. Specifically, we observe current densities of >8 mA/cm2 at -0.8 V vs the standard hydrogen electrode (SHE) by direct electron transfer (DET) from cyclic voltammetry, with an onset potential for H2 production close to its standard potential at pH 7 (approximately -0.4 V vs. SHE). Importantly, hydrogenase-modified electrodes show high stability retaining ∼92% of their electrocatalytic current after 120 h of continuous potentiostatic H2 production at -0.6 V vs. SHE; gas chromatography confirmed ∼100% Faradaic efficiency. As the bioelectrode preparation method balances simplicity, performance, and stability, it paves the way for DET on other electroenzymatic reactions as well as semiartificial photosynthesis.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Bermudez J.; Hasegawa T.; Bennett S.. Hydrogen – Analysis and Key Findings. A Report by the International Energy Agency. 2021. https://www.iea.org/reports/hydrogen (accessed 2022-04-20).
-
- Armstrong F. A.; Belsey N. A.; Cracknell J. A.; Goldet G.; Parkin A.; Reisner E.; Vincent K. A.; Wait A. F. Dynamic Electrochemical Investigations of Hydrogen Oxidation and Production by Enzymes and Implications for Future Technology. Chem. Soc. Rev. 2009, 38 (1), 36–51. 10.1039/B801144N. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
