This is a preprint.
Antimicrobial mitochondrial reactive oxygen species induction by lung epithelial metabolic reprogramming
- PMID: 36711510
- PMCID: PMC9882263
- DOI: 10.1101/2023.01.19.524841
Antimicrobial mitochondrial reactive oxygen species induction by lung epithelial metabolic reprogramming
Update in
-
Antimicrobial mitochondrial reactive oxygen species induction by lung epithelial immunometabolic modulation.PLoS Pathog. 2023 Sep 11;19(9):e1011138. doi: 10.1371/journal.ppat.1011138. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37695784 Free PMC article.
Abstract
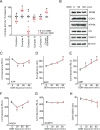
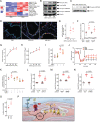
Pneumonia is a worldwide threat, making discovery of novel means to combat lower respiratory tract infections an urgent need. We have previously shown that manipulating the lungs' intrinsic host defenses by therapeutic delivery of a unique dyad of pathogen-associated molecular patterns protects mice against pneumonia in a reactive oxygen species (ROS)-dependent manner. Here we show that antimicrobial ROS are induced from lung epithelial cells by interactions of CpG oligodeoxynucleotides (ODNs) with mitochondrial voltage-dependent anion channel 1 (VDAC1) without dependence on Toll-like receptor 9 (TLR9). The ODN-VDAC1 interaction alters cellular ATP/ADP/AMP localization, increases delivery of electrons to the electron transport chain (ETC), enhances mitochondrial membrane potential (Δ Ψm ), and differentially modulates ETC complex activities. These combined effects promote leak of electrons from ETC complex III, resulting in superoxide formation. The ODN-induced mitochondrial ROS yield protective antibacterial effects. Together, these studies identify a therapeutic metabolic manipulation strategy that has the potential to broadly protect patients against pneumonia during periods of peak vulnerability without reliance on currently available antibiotics.
Author summary: Pneumonia is a major cause of death worldwide. Increasing antibiotic resistance and expanding immunocompromised populations continue to enhance the clinical urgency to find new strategies to prevent and treat pneumonia. We have identified a novel inhaled therapeutic that stimulates lung epithelial defenses to protect mice against pneumonia in a manner that depends on production of reactive oxygen species (ROS). Here, we report that the induction of protective ROS from lung epithelial mitochondria occurs following the interaction of one component of the treatment, an oligodeoxynucleotide, with the mitochondrial voltage-dependent anion channel 1. This interaction alters energy transfer between the mitochondria and the cytosol, resulting in metabolic reprogramming that drives more electrons into the electron transport chain, then causes electrons to leak from the electron transport chain to form protective ROS. While antioxidant therapies are endorsed in many other disease states, we present here an example of therapeutic induction of ROS that is associated with broad protection against pneumonia without reliance on administration of antibiotics.
Conflict of interest statement
Figures




References
-
- DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–344. - PMC - PubMed
-
- Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database Syst Rev. 2009(4):CD002109. - PubMed
-
- Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
