This is a preprint.
Conserved Roles for the Dynein Intermediate Chain and Ndel1 in Assembly and Activation of Dynein
- PMID: 36711700
- PMCID: PMC9882231
- DOI: 10.1101/2023.01.13.523097
Conserved Roles for the Dynein Intermediate Chain and Ndel1 in Assembly and Activation of Dynein
Update in
-
Conserved roles for the dynein intermediate chain and Ndel1 in assembly and activation of dynein.Nat Commun. 2023 Sep 20;14(1):5833. doi: 10.1038/s41467-023-41466-5. Nat Commun. 2023. PMID: 37730751 Free PMC article.
Abstract
Cytoplasmic dynein, the primary retrograde microtubule transport motor within cells, must be activated for processive motility through the regulated assembly of a dynein-dynactin-adapter (DDA) complex. The interaction between dynein and dynactin was initially ascribed to the N-terminus of the dynein intermediate chain (IC) and a coiled-coil of the dynactin subunit p150 Glued . However, cryo-EM structures of DDA complexes have not resolve these regions of the IC and p150 Glued , raising questions about the importance of this interaction. The IC N-terminus (ICN) also interacts with the dynein regulators Nde1/Ndel1, which compete with p150 Glued for binding to ICN. Using a combination of approaches, we reveal that the ICN plays critical, evolutionarily conserved roles in DDA assembly by interacting with dynactin and Ndel1, the latter of which recruits the DDA assembly factor LIS1 to the dynein complex. In contrast to prior models, we find that LIS1 cannot simultaneously bind to Ndel1 and dynein, indicating that LIS1 must be handed off from Ndel1 to dynein in temporally discrete steps. Whereas exogenous Ndel1 or p150 Glued disrupts DDA complex assembly in vitro , neither perturbs preassembled DDA complexes, indicating that the IC is stably bound to p150 Glued within activated DDA complexes. Our study reveals previously unknown regulatory steps in the dynein activation pathway, and provides a more complete model for how the activities of LIS1/Ndel1 and dynactin/cargo-adapters are integrated to regulate dynein motor activity.
Conflict of interest statement
Competing interest statement
The authors have no competing interests to declare.
Figures

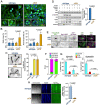


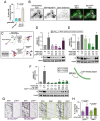

References
-
- Amos L.A. (1989). Brain dynein crossbridges microtubles into bundles. J Cell Sci 93, 19–28. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
