This is a preprint.
Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression
- PMID: 36711701
- PMCID: PMC9882616
- DOI: 10.21203/rs.3.rs-2318750/v1
Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression
Update in
-
Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression.Nat Commun. 2024 May 30;15(1):4099. doi: 10.1038/s41467-024-48441-8. Nat Commun. 2024. PMID: 38816352 Free PMC article.
Abstract
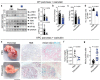
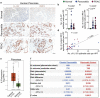
Chronic inflammation is a major cause of cancer worldwide. Interleukin 33 (IL-33) is a critical initiator of cancer-prone chronic inflammation; however, its induction mechanism by the environmental causes of chronic inflammation is unknown. Herein, we demonstrate that Toll-like receptor (TLR)3/4-TBK1-IRF3 pathway activation links environmental insults to IL-33 induction in the skin and pancreas. FDA-approved drug library screen identified pitavastatin as an effective IL-33 inhibitor by blocking TBK1 membrane recruitment/activation through the mevalonate pathway inhibition. Accordingly, pitavastatin prevented chronic pancreatitis and its cancer sequela in an IL-33-dependent manner. IRF3-IL-33 axis was highly active in chronic pancreatitis and its associated pancreatic cancer in humans. Interestingly, pitavastatin use correlated with a significantly reduced risk of chronic pancreatitis and pancreatic cancer in patients. Our findings demonstrate that blocking the TBK1-IRF3 signaling pathway suppresses IL-33 expression and cancer-prone chronic inflammation. Statins present a safe and effective therapeutic strategy to prevent chronic inflammation and its cancer sequela.
Keywords: Interleukin-33; TBK1-IRF3 pathway; cancer prevention; chronic inflammation; chronic pancreatitis; pancreatic cancer; pitavastatin.
Conflict of interest statement
COMPETING OF INTEREST JHP and SD are coinventors on a filed patent for the use of IL-33 inhibition in the treatment of cancer, fibrosis, and inflammation (PCT/US21/40725), which is licensed to DermBiont Inc. AM is an equity holder of DermBiont Inc. The remaining authors state no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

