This is a preprint.
Physical Activity Delays Obesity-Associated Pancreatic Ductal Adenocarcinoma in Mice and Decreases Inflammation
- PMID: 36711764
- PMCID: PMC9881853
- DOI: 10.1101/2023.01.03.521203
Physical Activity Delays Obesity-Associated Pancreatic Ductal Adenocarcinoma in Mice and Decreases Inflammation
Update in
-
Physical Activity Decreases Inflammation and Delays the Development of Obesity-Associated Pancreatic Ductal Adenocarcinoma.Cancer Res. 2024 Sep 16;84(18):3058-3071. doi: 10.1158/0008-5472.CAN-23-1045. Cancer Res. 2024. PMID: 38781455 Free PMC article.
Abstract
Background & aims: Obesity is a risk factor for pancreatic ductal adenocarcinoma (PDAC), a deadly disease with limited preventive strategies. Lifestyle interventions to decrease obesity might prevent obesity-associated PDAC. Here, we examined whether decreasing obesity by increased physical activity (PA) and/or dietary changes would decrease inflammation in humans and prevent PDAC in mice.
Methods: Circulating inflammatory-associated cytokines of overweight and obese subjects before and after a PA intervention were compared. PDAC pre-clinical models were exposed to PA and/or dietary interventions after obesity-associated cancer initiation. Body composition, tumor progression, growth, fibrosis, inflammation, and transcriptomic changes in the adipose tissue were evaluated.
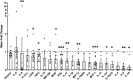
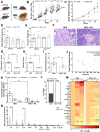
Results: PA decreased the levels of systemic inflammatory cytokines in overweight and obese subjects. PDAC mice on a diet-induced obesity (DIO) and PA intervention, had delayed weight gain, decreased systemic inflammation, lower grade pancreatic intraepithelial neoplasia lesions, reduced PDAC incidence, and increased anti-inflammatory signals in the adipose tissue compared to controls. PA had additional cancer prevention benefits when combined with a non-obesogenic diet after DIO. However, weight loss through PA alone or combined with a dietary intervention did not prevent tumor growth in an orthotopic PDAC model. Adipose-specific targeting of interleukin (IL)-15, an anti-inflammatory cytokine induced by PA in the adipose tissue, slowed PDAC growth.
Conclusions: PA alone or combined with diet-induced weight loss delayed the progression of PDAC and reduced systemic and adipose inflammatory signals. Therefore, obesity management via dietary interventions and/or PA, or modulating weight loss related pathways could prevent obesity-associated PDAC in high-risk obese individuals.
Keywords: Obesity; diet intervention; high-risk PDAC; voluntary wheel running; weight loss.
Conflict of interest statement
Conflict of interest/disclosures: None
Figures



References
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
