This is a preprint.
Magnetic seizure therapy and electroconvulsive therapy increase aperiodic activity
- PMID: 36711765
- PMCID: PMC9882553
- DOI: 10.1101/2023.01.11.23284450
Magnetic seizure therapy and electroconvulsive therapy increase aperiodic activity
Update in
-
Magnetic seizure therapy and electroconvulsive therapy increase aperiodic activity.Transl Psychiatry. 2023 Nov 16;13(1):347. doi: 10.1038/s41398-023-02631-y. Transl Psychiatry. 2023. PMID: 37968260 Free PMC article.
Abstract
Major depressive disorder (MDD) is a leading cause of disability worldwide. One of the most efficacious treatments for treatment-resistant MDD is electroconvulsive therapy (ECT). Recently, magnetic seizure therapy (MST) was developed as an alternative to ECT due to its more favorable side effect profile. While these approaches have been very successful clinically, the neural mechanisms underlying their therapeutic effects are unknown. For example, clinical "slowing" of the electroencephalogram beginning in the postictal state and extending days to weeks post-treatment has been observed in both treatment modalities. However, a recent longitudinal study of a small cohort of ECT patients revealed that, rather than delta oscillations, clinical slowing was better explained by increases in aperiodic activity, an emerging EEG signal linked to neural inhibition. Here we investigate the role of aperiodic activity in a cohort of patients who received ECT and a cohort of patients who received MST treatment. We find that aperiodic neural activity increases significantly in patients receiving either ECT or MST. Although not directly related to clinical efficacy in this dataset, increased aperiodic activity is linked to greater amounts of neural inhibition, which is suggestive of a potential shared neural mechanism of action across ECT and MST.
Conflict of interest statement
Competing interests A.T.H. was supported by an Alfred Deakin Postdoctoral Research Fellowship. D.M.B. receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health – US (NIH), Brain Canada Foundation and the Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he was the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen. He has participated in an advisory board for Welcony Inc. Z.J.D. has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and Magventure Inc and industry-initiated trials through Magnus Inc. He also currently serves on the scientific advisory board for Brainsway Inc. His work has been supported by the National Institutes of Mental Health (NIMH), the Canadian Institutes of Health Research (CIHR), Brain Canada and the Temerty Family, Grant and Kreutzcamp Family Foundations.
Figures


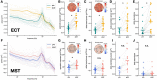


References
-
- The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. (American Psychiatric Association, 2001).
-
- UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. The Lancet 361, 799–808 (2003). - PubMed
-
- Lisanby S. H., Schlaepfer T. E., Fisch H.-U. & Sackeim H. A. Magnetic Seizure Therapy of Major Depression. Arch. Gen. Psychiatry 58, 303 (2001). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
