This is a preprint.
TGFB1 Induces Fetal Reprogramming and Enhances Intestinal Regeneration
- PMID: 36711781
- PMCID: PMC9882197
- DOI: 10.1101/2023.01.13.523825
TGFB1 Induces Fetal Reprogramming and Enhances Intestinal Regeneration
Update in
-
TGFB1 induces fetal reprogramming and enhances intestinal regeneration.Cell Stem Cell. 2023 Nov 2;30(11):1520-1537.e8. doi: 10.1016/j.stem.2023.09.015. Epub 2023 Oct 20. Cell Stem Cell. 2023. PMID: 37865088 Free PMC article.
Abstract
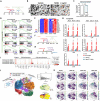
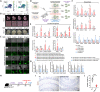
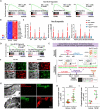
The adult gut epithelium has a remarkable ability to recover from damage. To achieve cellular therapies aimed at restoring and/or replacing defective gastrointestinal tissue, it is important to understand the natural mechanisms of tissue regeneration. We employed a combination of high throughput sequencing approaches, mouse genetic models, and murine and human organoid models, and identified a role for TGFB signaling during intestinal regeneration following injury. At 2 days following irradiation (IR)-induced damage of intestinal crypts, a surge in TGFB1 expression is mediated by monocyte/macrophage cells at the location of damage. Depletion of macrophages or genetic disruption of TGFB-signaling significantly impaired the regenerative response following irradiation. Murine intestinal regeneration is also characterized by a process where a fetal transcriptional signature is induced during repair. In organoid culture, TGFB1-treatment was necessary and sufficient to induce a transcriptomic shift to the fetal-like/regenerative state. The regenerative response was enhanced by the function of mesenchymal cells, which are also primed for regeneration by TGFB1. Mechanistically, integration of ATAC-seq, scRNA-seq, and ChIP-seq suggest that a regenerative YAP-SOX9 transcriptional circuit is activated in epithelium exposed to TGFB1. Finally, pre-treatment with TGFB1 enhanced the ability of primary epithelial cultures to engraft into damaged murine colon, suggesting promise for the application of the TGFB-induced regenerative circuit in cellular therapy.
Keywords: fetal reversion; intestine; regeneration.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare that they have no competing interests.
Figures




References
-
- Ayyaz A., Kumar S., Sangiorgi B., Ghoshal B., Gosio J., Ouladan S., Fink M., Barutcu S., Trcka D., Shen J., et al. (2019). Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
