This is a preprint.
Base editing as a genetic treatment for spinal muscular atrophy
- PMID: 36711797
- PMCID: PMC9882371
- DOI: 10.1101/2023.01.20.524978
Base editing as a genetic treatment for spinal muscular atrophy
Abstract
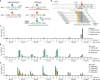
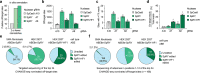
Spinal muscular atrophy (SMA) is a devastating neuromuscular disease caused by mutations in the SMN1 gene. Despite the development of various therapies, outcomes can remain suboptimal in SMA infants and the duration of such therapies are uncertain. SMN2 is a paralogous gene that mainly differs from SMN1 by a C•G-to-T•A transition in exon 7, resulting in the skipping of exon 7 in most SMN2 transcripts and production of only low levels of survival motor neuron (SMN) protein. Genome editing technologies targeted to the SMN2 exon 7 mutation could offer a therapeutic strategy to restore SMN protein expression to normal levels irrespective of the patient SMN1 mutation. Here, we optimized a base editing approach to precisely edit SMN2, reverting the exon 7 mutation via an A•T-to-G•C base edit. We tested a range of different adenosine base editors (ABEs) and Cas9 enzymes, resulting in up to 99% intended editing in SMA patient-derived fibroblasts with concomitant increases in SMN2 exon 7 transcript expression and SMN protein levels. We generated and characterized ABEs fused to high-fidelity Cas9 variants which reduced potential off-target editing. Delivery of these optimized ABEs via dual adeno-associated virus (AAV) vectors resulted in precise SMN2 editing in vivo in an SMA mouse model. This base editing approach to correct SMN2 should provide a long-lasting genetic treatment for SMA with advantages compared to current nucleic acid, small molecule, or exogenous gene replacement therapies. More broadly, our work highlights the potential of PAMless SpRY base editors to install edits efficiently and safely.
Keywords: CRISPR; SMA; adenine base editors (ABEs); genome editing; neuromuscular diseases.
Conflict of interest statement
Competing interests C.R.R.A., K.A.C., K.J.S., and B.P.K. are inventors on a patent application filed by Mass General Brigham (MGB) that describes genome engineering technologies to treat SMA. S.Q.T. and C.R.L are co-inventors on a patent application describing the CHANGE-seq method. S.Q.T. is a member of the scientific advisory board of Kromatid, Twelve Bio, and Prime Medicine. C.A.M. has a financial interest in Sphere Gene Therapeutics, Inc., Chameleon Biosciences, Inc., and Skylark Bio, Inc., companies developing gene therapy platforms. C.A.M.’s interests were reviewed and are managed by MGH and MGB in accordance with their conflict-of-interest policies. C.A.M. has a filed patent application with claims involving the AAV-F capsid. B.P.K. is an inventor on additional patents or patent applications filed by MGB that describe genome engineering technologies. B.P.K. is a consultant for EcoR1 capital and is on the scientific advisory board of Acrigen Biosciences, Life Edit Therapeutics, and Prime Medicine. S.Q.T. and B.P.K. have financial interests in Prime Medicine, Inc., a company developing therapeutic CRISPR-Cas technologies for gene editing. B.P.K.’s interests were reviewed and are managed by MGH and MGB in accordance with their conflict-of-interest policies. The other authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
