This is a preprint.
Neutrophil extracellular trap stabilization by platelet factor 4 reduces thrombogenicity and endothelial cell injury
- PMID: 36711969
- PMCID: PMC9881987
- DOI: 10.1101/2023.01.09.522931
Neutrophil extracellular trap stabilization by platelet factor 4 reduces thrombogenicity and endothelial cell injury
Update in
-
Platelet factor 4 limits neutrophil extracellular trap- and cell-free DNA-induced thrombogenicity and endothelial injury.JCI Insight. 2023 Nov 22;8(22):e171054. doi: 10.1172/jci.insight.171054. JCI Insight. 2023. PMID: 37991024 Free PMC article.
Abstract
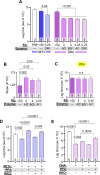
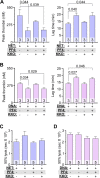
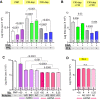
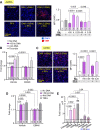
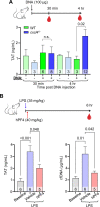
Neutrophil extracellular traps (NETs) are abundant in sepsis, and proposed NET-directed therapies in sepsis prevent their formation or accelerate degradation. Yet NETs are important for microbial entrapment, as NET digestion liberates pathogens and NET degradation products (NDPs) that deleteriously promote thrombosis and endothelial cell injury. We proposed an alternative strategy of NET-stabilization with the chemokine, platelet factor 4 (PF4, CXCL4), which we have shown enhances NET-mediated microbial entrapment. We now show that NET compaction by PF4 reduces their thrombogenicity. In vitro, we quantified plasma thrombin and fibrin generation by intact or degraded NETs and cell-free (cf) DNA fragments, and found that digested NETs and short DNA fragments were more thrombogenic than intact NETs and high molecular weight genomic DNA, respectively. PF4 reduced the thrombogenicity of digested NETs and DNA by interfering, in part, with contact pathway activation. In endothelial cell culture studies, short DNA fragments promoted von Willebrand factor release and tissue factor expression via a toll-like receptor 9-dependent mechanism. PF4 blocked these effects. Cxcl4-/- mice infused with cfDNA exhibited higher plasma thrombin anti-thrombin (TAT) levels compared to wild-type controls. Following challenge with bacterial lipopolysaccharide, Cxcl4-/- mice had similar elevations in plasma TAT and cfDNA, effects prevented by PF4 infusion. Thus, NET-stabilization by PF4 prevents the release of short fragments of cfDNA, limiting the activation of the contact coagulation pathway and reducing endothelial injury. These results support our hypothesis that NET-stabilization reduces pathologic sequelae in sepsis, an observation of potential clinical benefit.
Keywords: cell-free DNA; neutrophil extracellular trap; platelet factor 4; sepsis; thrombosis.
Conflict of interest statement
Conflict-of-interest disclosure The authors declare no conflict-of-interests.
Figures

References
-
- Jing Q, Leung CHC, Wu AR. Cell-Free DNA as Biomarker for Sepsis by Integration of Microbial and Host Information. Clin Chem. 2022;68(9):1184–1195. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
