This is a preprint.
Subclonal somatic copy number alterations emerge and dominate in recurrent osteosarcoma
- PMID: 36711976
- PMCID: PMC9881990
- DOI: 10.1101/2023.01.05.522765
Subclonal somatic copy number alterations emerge and dominate in recurrent osteosarcoma
Update in
-
Subclonal Somatic Copy-Number Alterations Emerge and Dominate in Recurrent Osteosarcoma.Cancer Res. 2023 Nov 15;83(22):3796-3812. doi: 10.1158/0008-5472.CAN-23-0385. Cancer Res. 2023. PMID: 37812025 Free PMC article.
Abstract
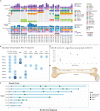
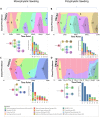
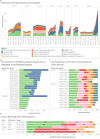
Multiple large-scale tumor genomic profiling efforts have been undertaken in osteosarcoma, however, little is known about the spatial and temporal intratumor heterogeneity and how it may drive treatment resistance. We performed whole-genome sequencing of 37 tumor samples from eight patients with relapsed or refractory osteosarcoma. Each patient had at least one sample from a primary site and a metastatic or relapse site. We identified subclonal copy number alterations in all but one patient. We observed that in five patients, a subclonal copy number clone from the primary tumor emerged and dominated at subsequent relapses. MYC gain/amplification was enriched in the treatment-resistant clone in 6 out of 7 patients with more than one clone. Amplifications in other potential driver genes, such as CCNE1, RAD21, VEGFA, and IGF1R, were also observed in the resistant copy number clones. Our study sheds light on intratumor heterogeneity and the potential drivers of treatment resistance in osteosarcoma.
Keywords: clonal evolution; intratumor heterogeneity; osteosarcoma; somatic copy number alterations; whole-genome sequencing.
Figures



References
-
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities or Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. Journal of Clinical Oncology. 2002;20(3):776–790. doi: 10.1200/jco.2002.20.3.776 - DOI - PubMed
-
- Sayles LC, Breese MR, Koehne AL, et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. Published online September 28, 2018:CD-17-1152. doi: 10.1158/2159-8290.CD-17-1152 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
