This is a preprint.
Spatial transcriptomics reveals unique gene expression changes in different brain regions after sleep deprivation
- PMID: 36712009
- PMCID: PMC9882298
- DOI: 10.1101/2023.01.18.524406
Spatial transcriptomics reveals unique gene expression changes in different brain regions after sleep deprivation
Update in
-
Spatial transcriptomics reveals unique gene expression changes in different brain regions after sleep deprivation.Nat Commun. 2023 Nov 4;14(1):7095. doi: 10.1038/s41467-023-42751-z. Nat Commun. 2023. PMID: 37925446 Free PMC article.
Abstract
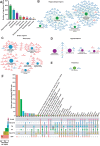
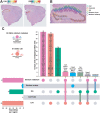
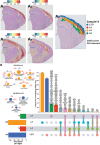
Sleep deprivation has far-reaching consequences on the brain and behavior, impacting memory, attention, and metabolism. Previous research has focused on gene expression changes in individual brain regions, such as the hippocampus or cortex. Therefore, it is unclear how uniformly or heterogeneously sleep loss affects the brain. Here, we use spatial transcriptomics to define the impact of a brief period of sleep deprivation across the brain. We find that sleep deprivation induced pronounced differences in gene expression across the brain, with the greatest changes in the hippocampus, neocortex, hypothalamus, and thalamus. Both the differentially expressed genes and the direction of regulation differed markedly across regions. Importantly, we developed bioinformatic tools to register tissue sections and gene expression data into a common anatomical space, allowing a brain-wide comparison of gene expression patterns between samples. Our results suggest that distinct molecular mechanisms acting in discrete brain regions underlie the biological effects of sleep deprivation.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures


References
-
- McHill A. W. & Wright K. P. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. Off. J. Int. Assoc. Study Obes. 18 Suppl 1, 15–24 (2017). - PubMed
-
- Raven F., Van der Zee E. A., Meerlo P. & Havekes R. The role of sleep in regulating structural plasticity and synaptic strength: Implications for memory and cognitive function. Sleep Med. Rev. 39, 3–11 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
