This is a preprint.
The DACH1 gene is frequently deleted in prostate cancer, restrains prostatic intraepithelial neoplasia, decreases DNA damage repair, and predicts therapy responses
- PMID: 36712010
- PMCID: PMC9882663
- DOI: 10.21203/rs.3.rs-2423179/v1
The DACH1 gene is frequently deleted in prostate cancer, restrains prostatic intraepithelial neoplasia, decreases DNA damage repair, and predicts therapy responses
Update in
-
The DACH1 gene is frequently deleted in prostate cancer, restrains prostatic intraepithelial neoplasia, decreases DNA damage repair, and predicts therapy responses.Oncogene. 2023 Jun;42(22):1857-1873. doi: 10.1038/s41388-023-02668-9. Epub 2023 Apr 24. Oncogene. 2023. PMID: 37095257 Free PMC article.
Abstract
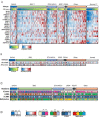
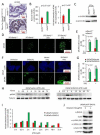
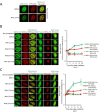
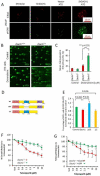
Prostate cancer (PCa), the second leading cause of death in American men, includes distinct genetic subtypes with distinct therapeutic vulnerabilities. The DACH1 gene encodes a winged helix/Forkhead DNA-binding protein that competes for binding to FOXM1 sites. Herein, DACH1 gene deletion within the 13q21.31-q21.33 region occurs in up to 18% of human PCa and was associated with increased AR activity and poor prognosis. In prostate OncoMice, prostate-specific deletion of the Dach1 gene enhanced prostatic intraepithelial neoplasia (PIN), and was associated with increased TGFb activity and DNA damage. Reduced Dach1 increased DNA damage in response to genotoxic stresses. DACH1 was recruited to sites of DNA damage, augmenting recruitment of Ku70/Ku80. Reduced Dach1 expression was associated with increased homology directed repair and resistance to PARP inhibitors and TGFb kinase inhibitors. Reduced Dach1 expression may define a subclass of PCa that warrants specific therapies.
Conflict of interest statement
CONFLICT OF INTEREST:
The authors declare that they have no relevant financial conflicts of interest. R.G.P. holds ownership interests in CytoDyn, EcoGenome, StromaGenesis, and LightSeed, Inc. R.G.P. additionally holds ownership interests (value unknown) for several patents and submitted patent applications.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

