This is a preprint.
Measuring prion propagation in single bacteria elucidates mechanism of loss
- PMID: 36712035
- PMCID: PMC9882039
- DOI: 10.1101/2023.01.11.523042
Measuring prion propagation in single bacteria elucidates mechanism of loss
Update in
-
Measuring prion propagation in single bacteria elucidates a mechanism of loss.Proc Natl Acad Sci U S A. 2023 Sep 26;120(39):e2221539120. doi: 10.1073/pnas.2221539120. Epub 2023 Sep 22. Proc Natl Acad Sci U S A. 2023. PMID: 37738299 Free PMC article.
Abstract
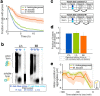
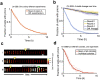
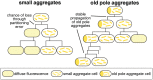
Prions are self-propagating protein aggregates formed by specific proteins that can adopt alternative folds. Prions were discovered as the cause of the fatal transmissible spongiform encephalopathies in mammals, but prions can also constitute non-toxic protein-based elements of inheritance in fungi and other species. Prion propagation has recently been shown to occur in bacteria for more than a hundred cell divisions, yet a fraction of cells in these lineages lost the prion through an unknown mechanism. Here, we investigate prion propagation in single bacterial cells as they divide using microfluidics and fluorescence microscopy. We show that the propagation occurs in two distinct modes with distinct stability and inheritance characteristics. We find that the prion is lost through random partitioning of aggregates to one of the two daughter cells at division. Extending our findings to prion domains from two orthologous proteins, we observe similar propagation and loss properties. Our findings also provide support for the suggestion that bacterial prions can form more than one self-propagating state. We implement a stochastic version of the molecular model of prion propagation from yeast and mammals that recapitulates all the observed single-cell properties. This model highlights challenges for prion propagation that are unique to prokaryotes and illustrates the conservation of fundamental characteristics of prion propagation across domains of life.
Figures



References
-
- Prusiner SB, Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982). - PubMed
-
- Glover JR, et al., Self-Seeded fibers formed by sup35, the protein determinant of [PSI+], a heritable prion-like factor of s. cerevisiae. Cell 89, 811–819 (1997). - PubMed
-
- True HL, Lindquist SL, A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407, 477–483 (2000). - PubMed
-
- Levkovich SA, Rencus-Lazar S, Gazit E, Bar-Yosef D Laor, Microbial prions: Dawn of a new era. Trends Biochem. Sci. 46, 391–405 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
