This is a preprint.
High Throughput Viability Assay for Microbiology
- PMID: 36712102
- PMCID: PMC9881960
- DOI: 10.1101/2023.01.04.522767
High Throughput Viability Assay for Microbiology
Update in
-
A high-throughput and low-waste viability assay for microbes.Nat Microbiol. 2023 Dec;8(12):2304-2314. doi: 10.1038/s41564-023-01513-9. Epub 2023 Nov 2. Nat Microbiol. 2023. PMID: 37919425 Free PMC article.
Abstract
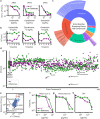
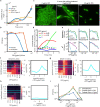
Counting viable cells is a universal practice in microbiology. The colony forming unit (CFU) assay has remained the gold standard to measure viability across disciplines; however, it is time-intensive and resource-consuming. Herein, we describe the Geometric Viability Assay (GVA) that replicates CFU measurements over 6-orders of magnitude while reducing over 10-fold the time and consumables. GVA computes a sample's viable cell count based on the distribution of embedded colonies growing inside a pipette tip. GVA is compatible with gram-positive and -negative planktonic bacteria, biofilms, and yeast. Laborious CFU experiments such as checkerboard assays, treatment time-courses, and drug screens against slow-growing cells are simplified by GVA. We therefore screened a drug library against exponential and stationary phase E. coli leading to the discovery of the ROS-mediated, bactericidal mechanism of diphenyliodonium. The ease and low cost of GVA evinces it can accelerate existing viability assays and enable measurements at previously impractical scales.
Conflict of interest statement
Declaration of Interests CTM and JMK have filed a provisional patent for the Geometric Viability Assay. CTM is a co-founder of Duet BioSystems. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Zheng E. J., Stokes J. M. & Collins J. J. Eradicating Bacterial Persisters with Combinations of Strongly and Weakly Metabolism-Dependent Antibiotics. Cell chemical biology 27, 1544–1552 (Dec. 2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
