Sex differences in interindividual gene expression variability across human tissues
- PMID: 36712323
- PMCID: PMC9802459
- DOI: 10.1093/pnasnexus/pgac243
Sex differences in interindividual gene expression variability across human tissues
Abstract
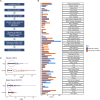
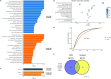
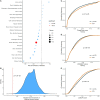
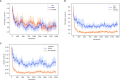
Understanding phenotypic sex differences has long been a goal of biology from both a medical and evolutionary perspective. Although much attention has been paid to mean differences in phenotype between the sexes, little is known about sex differences in phenotypic variability. To gain insight into sex differences in interindividual variability at the molecular level, we analyzed RNA-seq data from 43 tissues from the Genotype-Tissue Expression project (GTEx). Within each tissue, we identified genes that show sex differences in gene expression variability. We found that these sex-differentially variable (SDV) genes are associated with various important biological functions, including sex hormone response, immune response, and other signaling pathways. By analyzing single-cell RNA sequencing data collected from breast epithelial cells, we found that genes with sex differences in gene expression variability in breast tissue tend to be expressed in a cell-type-specific manner. We looked for an association between SDV expression and Graves' disease, a well-known heavily female-biased disease, and found a significant enrichment of Graves' associated genes among genes with higher variability in females in thyroid tissue. This suggests a possible role for SDV expression in sex-biased disease. We then examined the evolutionary constraints acting on genes with sex differences in variability and found that they exhibit evidence of increased selective constraint. Through analysis of sex-biased eQTL data, we found evidence that SDV expression may have a genetic basis. Finally, we propose a simple evolutionary model for the emergence of SDV expression from sex-specific constraints.
© The Author(s) 2022. Published by Oxford University Press on behalf of the National Academy of Sciences.
Figures
Similar articles
-
Regulatory and evolutionary signatures of sex-biased genes on both the X chromosome and the autosomes.Biol Sex Differ. 2017 Nov 2;8(1):35. doi: 10.1186/s13293-017-0156-4. Biol Sex Differ. 2017. PMID: 29096703 Free PMC article.
-
Genetic factors contributing to extensive variability of sex-specific hepatic gene expression in Diversity Outbred mice.PLoS One. 2020 Dec 2;15(12):e0242665. doi: 10.1371/journal.pone.0242665. eCollection 2020. PLoS One. 2020. PMID: 33264334 Free PMC article.
-
Sex-dependent gene co-expression in the human body.Sci Rep. 2021 Sep 21;11(1):18758. doi: 10.1038/s41598-021-98059-9. Sci Rep. 2021. PMID: 34548535 Free PMC article.
-
The evolution of sex-biased genes and sex-biased gene expression.Nat Rev Genet. 2007 Sep;8(9):689-98. doi: 10.1038/nrg2167. Epub 2007 Aug 7. Nat Rev Genet. 2007. PMID: 17680007 Review.
-
Stochastic developmental variation, an epigenetic source of phenotypic diversity with far-reaching biological consequences.J Biosci. 2015 Mar;40(1):159-204. doi: 10.1007/s12038-015-9506-8. J Biosci. 2015. PMID: 25740150 Review.
Cited by
-
Sex matters: the frequently overlooked importance of considering sex in computational models.Front Physiol. 2023 Jul 13;14:1186646. doi: 10.3389/fphys.2023.1186646. eCollection 2023. Front Physiol. 2023. PMID: 37520817 Free PMC article. Review.
-
Dietary restriction reveals sex-specific expression of the mTOR pathway genes in Japanese quails.Sci Rep. 2024 Apr 9;14(1):8314. doi: 10.1038/s41598-024-58487-9. Sci Rep. 2024. PMID: 38594358 Free PMC article.
-
Sex-based disparities in DNA methylation and gene expression in late-gestation mouse placentas.Biol Sex Differ. 2024 Jan 6;15(1):2. doi: 10.1186/s13293-023-00577-w. Biol Sex Differ. 2024. PMID: 38183126 Free PMC article.
-
ComBatLS: A Location- and Scale-Preserving Method for Multi-Site Image Harmonization.Hum Brain Mapp. 2025 Jun 1;46(8):e70197. doi: 10.1002/hbm.70197. Hum Brain Mapp. 2025. PMID: 40497521 Free PMC article.
-
Paternal impact on the developmental programming of sexual dimorphism.Front Cell Dev Biol. 2024 Dec 6;12:1520783. doi: 10.3389/fcell.2024.1520783. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39712575 Free PMC article. Review.
References
-
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol. 16: 626–638. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

