Inhibitory effect of phytochemicals towards SARS-CoV-2 papain like protease (PLpro) proteolytic and deubiquitinase activity
- PMID: 36712981
- PMCID: PMC9878345
- DOI: 10.3389/fchem.2022.1100460
Inhibitory effect of phytochemicals towards SARS-CoV-2 papain like protease (PLpro) proteolytic and deubiquitinase activity
Abstract
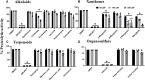
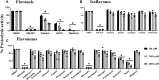
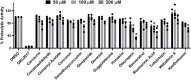
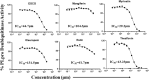
Recent studies have shown that RNA-dependent RNA polymerase (RdRp), 3-chymotrypsin-like protease (3CLpro), and papain-like protease (PLpro) are necessary for SARS-CoV-2 replication. Among these three enzymes, PLpro exhibits both proteolytic and deubiquitinase (DUB) activity and is responsible for disrupting the host's innate immune response against SARS-CoV-2. Because of this unique property of PLpro, we investigated the inhibitory effects of phytochemicals on the SARS-CoV-2 PLpro enzyme. Our data indicates that the phytochemicals such as catechin, epigallocatechin gallate (EGCG), mangiferin, myricetin, rutin, and theaflavin exhibited inhibitory activity with IC50 values of 14.2, 128.4, 95.3, 12.1, and 43.4, and 7.3 μM, respectively, towards PLpro proteolytic activity. However, the IC50 values of quercetin, oleuropein, and γ-mangostin are ambiguous. We observed that EGCG, mangiferin, myricetin, oleuropein, rutin, and theaflavin have also inhibited the DUB activity with IC50 values of 44.7, 104.3, 29.2, 131.5, 61.7, and 13.2 μM, respectively. Mechanistically, the ligand-protein interaction structural modeling suggests that mangiferin, EGCG, theaflavin, and oleuropein shows that these four ligands interact with Glu167, and Tyr268, however mangiferin and oleuropein showed very weak interaction with Glu167 as compared to EGCG, and theaflavin which reflects their low IC50 values for DUB activity. Our data indicate that the phytochemicals mentioned above inhibit the proteolytic and DUB activity of SARS-CoV-2 PLpro, thus preventing viral replication and promoting host innate immune response. However, the therapeutic potential of these phytochemicals needs to be validated by pre-clinical and clinical studies.
Keywords: PLpro; SARS-CoV-2; natural compounds; phytochemicals; replication.
Copyright © 2023 Kawall, Lewis, Sharma, Chavada, Deshmukh, Rayalam, Mody and Taval.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

