Microbiota from Exercise Mice Counteracts High-Fat High-Cholesterol Diet-Induced Cognitive Impairment in C57BL/6 Mice
- PMID: 36713033
- PMCID: PMC9883105
- DOI: 10.1155/2023/2766250
Microbiota from Exercise Mice Counteracts High-Fat High-Cholesterol Diet-Induced Cognitive Impairment in C57BL/6 Mice
Abstract
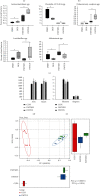
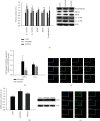
Gut microbes may be the critical mediators for the cognitive enhancing effects of exercise. Via fecal microbiota transplantation (FMT), this study is aimed at determining the mechanism of how voluntary exercise improved learning and memory ability impairment post a high-fat, high-cholesterol (HFHC) diet. The learning and memory abilities assessed via the Morris water maze in the FMT recipient group of voluntary exercising mice were improved compared to sedentary group. 16S rRNA gene sequencing results indicated that exercise-induced changes in gut microbiota distribution were transmissible, mainly in terms of elevated Lactobacillus, Lactobacillus, and Eubacterium nodatum, as well as decreased Clostrida_UCG-014 and Akkermansia after FMT. The neuroprotective effects of FMT were mainly related to the improved insulin signaling pathway (IRS2/PI3K/AKT) and mitochondrial function; inhibition of AQP4; decreased p-Tau at serine 396 and 404; increased BDNF, PSD95, and synaptophysin in the hippocampus; and also decreased HDAC2 and HDAC3 protein expressions in the nuclear and cytoplasmic fractions of the hippocampus. The findings of qRT-PCR suggested that exercise-induced gut microbes, on the one hand, elevated GPR109A and decreased GPR43 and TNF-α in the hippocampus. On the other hand, it increased GPR109A and GPR41 expressions in the proximal colon tissue. In addition, total short-chain fatty acid (SCFA), acetic acid, propionic acid, isobutyric acid, valeric acid, and isovaleric acid contents were also elevated in the cecum. In conclusion, exercise-induced alterations in gut microbiota play a decisive role in ameliorating HFHC diet-induced cognitive deficits. FMT treatment may be a new considerable direction in ameliorating cognitive impairment induced by exposure to HFHC diet.
Copyright © 2023 Rui Li et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

