Marine vampires: Persistent, internal associations between bacteria and blood-feeding marine annelids and crustaceans
- PMID: 36713196
- PMCID: PMC9876621
- DOI: 10.3389/fmicb.2022.1113237
Marine vampires: Persistent, internal associations between bacteria and blood-feeding marine annelids and crustaceans
Abstract
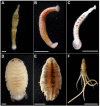
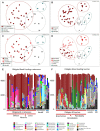
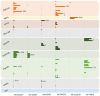
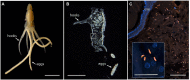
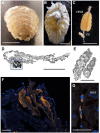
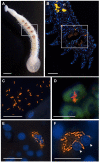
Persistent bacterial presence is believed to play an important role in host adaptation to specific niches that would otherwise be unavailable, including the exclusive consumption of blood by invertebrate parasites. Nearly all blood-feeding animals examined so far host internal bacterial symbionts that aid in some essential aspect of their nutrition. Obligate blood-feeding (OBF) invertebrates exist in the oceans, yet symbiotic associations between them and beneficial bacteria have not yet been explored. This study describes the microbiome of 6 phylogenetically-diverse species of marine obligate blood-feeders, including leeches (both fish and elasmobranch specialists; e.g., Pterobdella, Ostreobdella, and Branchellion), isopods (e.g., Elthusa and Nerocila), and a copepod (e.g., Lernanthropus). Amplicon sequencing analysis revealed the blood-feeding invertebrate microbiomes to be low in diversity, compared to host fish skin surfaces, seawater, and non-blood-feeding relatives, and dominated by only a few bacterial genera, including Vibrio (100% prevalence and comprising 39%-81% of the average total recovered 16S rRNA gene sequences per OBF taxa). Vibrio cells were localized to the digestive lumen in and among the blood meal for all taxa examined via fluorescence microscopy. For Elthusa and Branchellion, Vibrio cells also appeared intracellularly within possible hemocytes, suggesting an interaction with the immune system. Additionally, Vibrio cultivated from four of the obligate blood-feeding marine taxa matched the dominant amplicons recovered, and all but one was able to effectively lyse vertebrate blood cells. Bacteria from 2 additional phyla and 3 families were also regularly recovered, albeit in much lower abundances, including members of the Oceanospirillaceae, Flavobacteriacea, Porticoccaceae, and unidentified members of the gamma-and betaproteobacteria, depending on the invertebrate host. For the leech Pterobdella, the Oceanospirillaceae were also detected in the esophageal diverticula. For two crustacean taxa, Elthusa and Lernanthropus, the microbial communities associated with brooded eggs were very similar to the adults, indicating possible direct transmission. Virtually nothing is known about the influence of internal bacteria on the success of marine blood-feeders, but this evidence suggests their regular presence in marine parasites from several prominent groups.
Keywords: Vibrio; hematophagous; marine leech; obligate blood-feeding; parasitic copepod; parasitic isopod; symbiosis.
Copyright © 2023 Goffredi, Appy, Hildreth and deRogatis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Boxshall G. A. (1986). A new genus and two new species of Pennellidae (Copepoda: Siphonostomatoida) and an analysis of evolution within the family. Syst. Parasitol. 8, 215–225. doi: 10.1007/BF00009890 - DOI
-
- Brusca R. C. (1978). Studies on the Cymothoid fish symbionts of the eastern Pacific (isopoda, Cymothoidae) I Biology of Nerocila californica. Crustaceana 34, 141–154.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

