Application of individualized multimodal radiotherapy combined with immunotherapy in metastatic tumors
- PMID: 36713375
- PMCID: PMC9877461
- DOI: 10.3389/fimmu.2022.1106644
Application of individualized multimodal radiotherapy combined with immunotherapy in metastatic tumors
Abstract
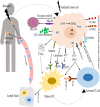
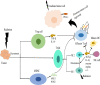
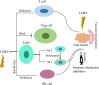
Radiotherapy is one of the mainstays of cancer treatment. More than half of cancer patients receive radiation therapy. In addition to the well-known direct tumoricidal effect, radiotherapy has immunomodulatory properties. When combined with immunotherapy, radiotherapy, especially high-dose radiotherapy (HDRT), exert superior systemic effects on distal and unirradiated tumors, which is called abscopal effect. However, these effects are not always effective for cancer patients. Therefore, many studies have focused on exploring the optimized radiotherapy regimens to further enhance the antitumor immunity of HDRT and reduce its immunosuppressive effect. Several studies have shown that low-dose radiotherapy (LDRT) can effectively reprogram the tumor microenvironment, thereby potentially overcoming the immunosuppressive stroma induced by HDRT. However, bridging the gap between preclinical commitment and effective clinical delivery is challenging. In this review, we summarized the existing studies supporting the combined use of HDRT and LDRT to synergistically enhance antitumor immunity, and provided ideas for the individualized clinical application of multimodal radiotherapy (HDRT+LDRT) combined with immunotherapy.
Keywords: cancer; high-dose radiotherapy; immunotherapy; low-dose radiotherapy; metastatic tumor.
Copyright © 2023 Ji, Jiang, Wang, Zhou, Ding, Liu, Huang, Chen and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Low-dose radiation therapy mobilizes antitumor immunity: New findings and future perspectives.Int J Cancer. 2024 Apr 1;154(7):1143-1157. doi: 10.1002/ijc.34801. Epub 2023 Dec 7. Int J Cancer. 2024. PMID: 38059788 Review.
-
A 'Hybrid' Radiotherapy Regimen Designed for Immunomodulation: Combining High-Dose Radiotherapy with Low-Dose Radiotherapy.Cancers (Basel). 2022 Jul 19;14(14):3505. doi: 10.3390/cancers14143505. Cancers (Basel). 2022. PMID: 35884565 Free PMC article. Review.
-
Combining immunotherapy with high-dose radiation therapy (HDRT) significantly inhibits tumor growth in a syngeneic mouse model of high-risk neuroblastoma.Heliyon. 2023 Jun 19;9(6):e17399. doi: 10.1016/j.heliyon.2023.e17399. eCollection 2023 Jun. Heliyon. 2023. PMID: 37408891 Free PMC article.
-
Effect of Low-Dose Radiation Therapy on Abscopal Responses to Hypofractionated Radiation Therapy and Anti-PD1 in Mice and Patients With Non-Small Cell Lung Cancer.Int J Radiat Oncol Biol Phys. 2020 Sep 1;108(1):212-224. doi: 10.1016/j.ijrobp.2020.05.002. Epub 2020 May 15. Int J Radiat Oncol Biol Phys. 2020. PMID: 32417411
-
Exploring low-dose radiotherapy to overcome radio-immunotherapy resistance.Biochim Biophys Acta Mol Basis Dis. 2023 Oct;1869(7):166789. doi: 10.1016/j.bbadis.2023.166789. Epub 2023 Jun 10. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 37302425 Review.
Cited by
-
Expanding horizons in esophageal squamous cell carcinoma: The promise of induction chemoimmunotherapy with radiotherapy.World J Clin Oncol. 2025 Jul 24;16(7):104959. doi: 10.5306/wjco.v16.i7.104959. World J Clin Oncol. 2025. PMID: 40741200 Free PMC article.
-
Immune modulatory roles of radioimmunotherapy: biological principles and clinical prospects.Front Immunol. 2024 Feb 21;15:1357101. doi: 10.3389/fimmu.2024.1357101. eCollection 2024. Front Immunol. 2024. PMID: 38449871 Free PMC article.
-
Regorafenib alone or in combination with high/low-dose radiotherapy plus toripalimab as third-line treatment in patients with metastatic colorectal cancer: protocol for a prospective, randomized, controlled phase II clinical trial (SLOT).Front Oncol. 2023 Oct 5;13:1274487. doi: 10.3389/fonc.2023.1274487. eCollection 2023. Front Oncol. 2023. PMID: 37869085 Free PMC article.
-
Hacking the Immune Response to Solid Tumors: Harnessing the Anti-Cancer Capacities of Oncolytic Bacteria.Pharmaceutics. 2023 Jul 21;15(7):2004. doi: 10.3390/pharmaceutics15072004. Pharmaceutics. 2023. PMID: 37514190 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

