Robust humoral and cellular recall responses to AZD1222 attenuate breakthrough SARS-CoV-2 infection compared to unvaccinated
- PMID: 36713413
- PMCID: PMC9881590
- DOI: 10.3389/fimmu.2022.1062067
Robust humoral and cellular recall responses to AZD1222 attenuate breakthrough SARS-CoV-2 infection compared to unvaccinated
Abstract
Background: Breakthrough severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in coronavirus disease 2019 (COVID-19) vaccinees typically produces milder disease than infection in unvaccinated individuals.
Methods: To explore disease attenuation, we examined COVID-19 symptom burden and immuno-virologic responses to symptomatic SARS-CoV-2 infection in participants (AZD1222: n=177/17,617; placebo: n=203/8,528) from a 2:1 randomized, placebo-controlled, phase 3 study of two-dose primary series AZD1222 (ChAdOx1 nCoV-19) vaccination (NCT04516746).
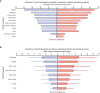
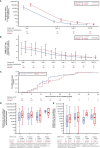
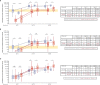
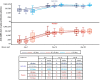
Results: We observed that AZD1222 vaccinees had an overall lower incidence and shorter duration of COVID-19 symptoms compared with placebo recipients, as well as lower SARS-CoV-2 viral loads and a shorter median duration of viral shedding in saliva. Vaccinees demonstrated a robust antibody recall response versus placebo recipients with low-to-moderate inverse correlations with virologic endpoints. Vaccinees also demonstrated an enriched polyfunctional spike-specific Th-1-biased CD4+ and CD8+ T-cell response that was associated with strong inverse correlations with virologic endpoints.
Conclusion: Robust immune responses following AZD1222 vaccination attenuate COVID-19 disease severity and restrict SARS-CoV-2 transmission potential by reducing viral loads and the duration of viral shedding in saliva. Collectively, these analyses underscore the essential role of vaccination in mitigating the COVID-19 pandemic.
Keywords: AZD1222 (ChAdOx1 nCoV-19); COVID-19 vaccine; SARS-CoV-2; breakthrough infection; cell-mediated immunity; serology.
Copyright © 2023 Maaske, Sproule, Falsey, Sobieszczyk, Luetkemeyer, Paulsen, Riddler, Robb, Rolle, Sha, Tong, Ahani, Aksyuk, Bansal, Egan, Jepson, Padilla, Patel, Shoemaker, Stanley, Swanson, Wilkins, Villafana, Green and Kelly.
Conflict of interest statement
JM, BA, AAA, TE, KS, AMS, MP, PAS, DW, TV, JAG, and EJK are current employees of AstraZeneca and hold or may hold AstraZeneca stock. HB and NP are contractors to AstraZeneca via Bogier consulting. BJ is a contractor to AstraZeneca via Cytel. SS is a contractor to AstraZeneca via Joule/System One. ARF has received institutional grants for research from Pfizer, Merck, Sharpe and Dohme, Janssen, and BioFire Diagnostics and has received fees for serving on Novavax COVID-19 vaccine Data and Safety Monitoring Board. MES declares grants from the NIH and NIAID during the conduct of the study and institutional research grants from the Bill and Melinda Gates Foundation, Gilead Sciences, Janssen Global Services, LLC, Merck, and Sanofi Pasteur Inc. AFL has received institutional research grants from AstraZeneca and Gilead Sciences. GCP has received institutional research grants from AstraZeneca, Pfizer, and Moderna. SAR has received institutional grants from the NIH and NIAID from clinical trial enrolment and provides pro bono consultancy to Novimmune for novimab. MLR declares funding for consultancy from the Walter Reed Army Institute of Research and for serving on their behalf in Operation Warp Speed. C-PR has received institutional research grants from Gilead Sciences and ViiV Healthcare, honoraria for lectures from Gilead Sciences, ViiV Healthcare and Vindico CME, and sits on advisory boards for Janssen, Gilead Sciences, and ViiV Healthcare. BES has received research funding from the US government under the COVID-19 Prevention Network initiative, institutional research grants from Gilead Sciences, ViiV Healthcare, and the University of Chicago and payment or honoraria from MATEC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This study received funding from AstraZeneca. The funder had the following involvement with the study with input from the external authors: study design; collection, analysis and interpretation of data; the writing of this article and the decision to submit it for publication. The first draft of the manuscript was written under the direction of the authors by a medical writer funded by AstraZeneca.
Figures



References
-
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-Biontech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ (2021) 373:n1088. doi: 10.1136/bmj.n1088 - DOI - PMC - PubMed
-
- World Health Organization (WHO) . WHO Coronavirus (COVID-19) dashboard. Available at: https://COVID19.Who.Int/ (Accessed 26 Aug 2022).
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- UM1 AI148372/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI148574/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

