Human pegivirus-1 replication influences NK cell reconstitution after allogeneic haematopoietic stem cell transplantation
- PMID: 36713419
- PMCID: PMC9876574
- DOI: 10.3389/fimmu.2022.1060886
Human pegivirus-1 replication influences NK cell reconstitution after allogeneic haematopoietic stem cell transplantation
Erratum in
-
Erratum: Human pegivirus-1 replication influences NK cell reconstitution after allogeneic haematopoietic stem cell transplantation.Front Immunol. 2023 Mar 6;14:1170106. doi: 10.3389/fimmu.2023.1170106. eCollection 2023. Front Immunol. 2023. PMID: 36949941 Free PMC article.
Abstract
Introduction: Human pegivirus-1 (HPgV-1) is a so-called commensal virus for which no known associated organ disease has been found to date. Yet, it affects immune-reconstitution as previously studied in the HIV population, in whom active co-infection with HPgV-1 can modulate T and NK cell activation and differentiation leading to a protective effect against the evolution of the disease. Little is known on the effect of HPgV-1 on immune-reconstitution in allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients, a patient population in which we and others have previously reported high prevalence of HPgV-1 replication. The aim of this study was to compare the immune reconstitution after allo-HSCT among HPgV-1-viremic and HPgV-1-non-viremic patients.
Methods: Within a cohort study of 40 allo-HSCT patients, 20 allo-HSCT recipients positive in plasma sample for HPgV-1 by rRT-PCR during the first year (1, 3, 6, 12 months) after transplantation were matched with 20 allo-HSCT recipients negative for HPgV-1. T and NK cell reconstitution was monitored by flow cytometry in peripheral blood samples from allo-HSCT recipients at the same time points.
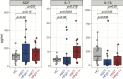
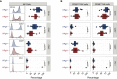
Results: We observed no significant difference in the absolute number and subsets proportions of CD4 and CD8 T cells between patient groups at any analysed timepoint. We observed a significantly higher absolute number of NK cells at 3 months among HPgV-1-viremic patients. Immunophenotypic analysis showed a significantly higher proportion of CD56bright NK cells mirrored by a reduced percentage of CD56dim NK cells in HPgV-1-positive patients during the first 6 months after allo-HSCT. At 6 months post-allo-HSCT, NK cell phenotype significantly differed depending on HPgV-1, HPgV-1-viremic patients displaying NK cells with lower CD16 and CD57 expression compared with HPgV-1-negative patients. In accordance with their less differentiated phenotype, we detected a significantly reduced expression of granzyme B in NK cells in HPgV-1-viremic patients at 6 months.
Discussion: Our study shows that HPgV-1-viremic allo-HSCT recipients displayed an impaired NK cell, but not T cell, immune-reconstitution compared with HPgV-1-non-viremic patients, revealing for the first time a potential association between replication of the non-pathogenic HPgV-1 virus and immunomodulation after allo-HSCT.
Keywords: CD16; CD57; NK cell; granzyme B; human pegivirus-1; stem cell; transplantation.
Copyright © 2023 Pradier, Cordey, Zanella, Melotti, Wang, Mamez, Chalandon, Masouridi-Levrat, Kaiser, Simonetta and Vu.
Conflict of interest statement
YC: consulting fees from MSD, Novartis, Incyte, BMS, Pfizer, Abbvie, Roche, Jazz, Gilead, Amgen, Astra-Zeneca, Servier; Travel support from MSD, Roche, Gilead, Amgen, Incyte, Abbvie, Janssen, Astra-Zeneca, Jazz. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

