Research progress on circular RNA vaccines
- PMID: 36713460
- PMCID: PMC9878156
- DOI: 10.3389/fimmu.2022.1091797
Research progress on circular RNA vaccines
Abstract
Owing to the success of linear mRNA coronavirus disease 2019 (COVID-19) vaccines, biopharmaceutical companies and research teams worldwide have attempted to develop more stable circular RNA (circRNA) vaccines and have achieved some preliminary results. This review aims to summarize key findings and important progress made in circRNA research, the in vivo metabolism and biological functions of circRNAs, and research progress and production process of circRNA vaccines. Further, considerations regarding the quality control of circRNA vaccines are highlighted herein, and the main challenges and problem-solving strategies in circRNA vaccine development and quality control are outlined to provide a reference for circRNA vaccine-related research.
Keywords: circular RNA vaccines; outlook; production process; quality control; research progress.
Copyright © 2023 Bai, Liu, He, Liu, Mao and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

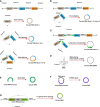
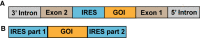
Similar articles
-
Advances in Engineering Circular RNA Vaccines.Pathogens. 2024 Aug 15;13(8):692. doi: 10.3390/pathogens13080692. Pathogens. 2024. PMID: 39204292 Free PMC article. Review.
-
Expanding the Potential of Circular RNA (CircRNA) Vaccines: A Promising Therapeutic Approach.Int J Mol Sci. 2025 Jan 4;26(1):379. doi: 10.3390/ijms26010379. Int J Mol Sci. 2025. PMID: 39796233 Free PMC article. Review.
-
Circular RNA vaccines against SARS-CoV-2 and emerging variants.Cell. 2022 May 12;185(10):1728-1744.e16. doi: 10.1016/j.cell.2022.03.044. Epub 2022 Apr 1. Cell. 2022. PMID: 35460644 Free PMC article.
-
Vaccines' New Era-RNA Vaccine.Viruses. 2023 Aug 18;15(8):1760. doi: 10.3390/v15081760. Viruses. 2023. PMID: 37632102 Free PMC article. Review.
-
Research progress on the quality control of mRNA vaccines.Expert Rev Vaccines. 2024 Jan-Dec;23(1):570-583. doi: 10.1080/14760584.2024.2354251. Epub 2024 May 24. Expert Rev Vaccines. 2024. PMID: 38733272 Review.
Cited by
-
RNA vaccines: The dawn of a new age for tuberculosis?Hum Vaccin Immunother. 2025 Dec;21(1):2469333. doi: 10.1080/21645515.2025.2469333. Epub 2025 Feb 27. Hum Vaccin Immunother. 2025. PMID: 40013818 Free PMC article. Review.
-
Promising Vaccine Formulations for Emerging Infectious Diseases.Int J Mol Sci. 2025 May 20;26(10):4893. doi: 10.3390/ijms26104893. Int J Mol Sci. 2025. PMID: 40430032 Free PMC article. Review.
-
mRNA vaccine technology for infectious diseases and beyond.Sci China Life Sci. 2024 Oct;67(10):2267-2270. doi: 10.1007/s11427-024-2639-3. Epub 2024 Jul 1. Sci China Life Sci. 2024. PMID: 38965140 No abstract available.
-
A Novel Targeted RIG-I Receptor 5'Triphosphate Double Strain RNA-Based Adjuvant Significantly Improves the Immunogenicity of the SARS-CoV-2 Delta-Omicron Chimeric RBD-Dimer Recombinant Protein Vaccine.Viruses. 2023 Apr 29;15(5):1099. doi: 10.3390/v15051099. Viruses. 2023. PMID: 37243185 Free PMC article.
-
Circular RNAs: Novel Players in Cancer Mechanisms and Therapeutic Strategies.Int J Mol Sci. 2024 Sep 20;25(18):10121. doi: 10.3390/ijms251810121. Int J Mol Sci. 2024. PMID: 39337606 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

