Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances
- PMID: 36713461
- PMCID: PMC9874322
- DOI: 10.3389/fimmu.2022.1063543
Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances
Abstract
Objective: Sepsis is the leading cause of death in critically ill patients. The gastrointestinal tract has long been thought to play an important role in the pathophysiology of sepsis. Antibiotic therapy can reduce a patient's commensal bacterial population and raise their risk of developing subsequent illnesses, where gut microbiota dysbiosis may be a key factor.
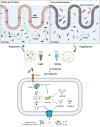
Methods: In this study, we analyzed the 16S rRNA of fecal samples from both healthy people and patients with sepsis to determine if alterations in gut bacteria are associated with sepsis. Then, we developed a mouse model of sepsis using cecal ligation and puncture (CLP) in order to examine the effects of fecal microbiota transplantation (FMT) and short-chain fatty acids (SCFAs) on survival rate, systemic inflammatory response, gut microbiota, and mucosal barrier function.
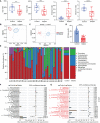
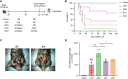
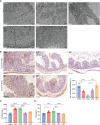
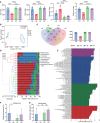
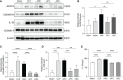
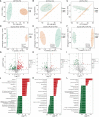
Results: Sepsis patients' gut microbiota composition significantly differed from that of healthy people. At the phylum level, the amount of Proteobacteria in the intestinal flora of sepsis patients was much larger than that of the control group, whereas the number of Firmicutes was significantly lower. Mice with gut microbiota disorders (ANC group) were found to have an elevated risk of death, inflammation, and organ failure as compared to CLP mice. However, all of these could be reversed by FMT and SCFAs. FMT and SCFAs could regulate the abundance of bacteria such as Firmicutes, Proteobacteria, Escherichia Shigella, and Lactobacillus, restoring them to levels comparable to those of healthy mice. In addition, they increased the expression of the Occludin protein in the colon of mice with sepsis, downregulated the expression of the NLRP3 and GSDMD-N proteins, and reduced the release of the inflammatory factors IL-1β and IL-18 to inhibit cell pyroptosis, ultimately playing a protective role in sepsis.
Disccusion: FMT and SCFAs provide a microbe-related survival benefit in a mouse model of sepsis, suggesting that they may be a viable treatment for sepsis.
Keywords: antibiotic; fecal microbiota transplantation; gut microbiota; sepsis; short-chain fatty acids.
Copyright © 2023 Lou, Xue, Shao, Yang, Ning, Mo, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

