Defining biomarkers in oral cancer according to smoking and drinking status
- PMID: 36713516
- PMCID: PMC9875375
- DOI: 10.3389/fonc.2022.1068979
Defining biomarkers in oral cancer according to smoking and drinking status
Abstract
Introduction: Oral Squamous Cell Carcinomas (OSCC) are mostly related to tobacco consumption eventually associated to alcohol (Smoker/Drinker patients: SD), but 25-30% of the patients have no identified risk factors (Non-Smoker/Non-Drinker patients: NSND). We hypothesized that these patients have distinguishable immune profiles that could be useful for prognosis.
Materials and methods: Cells present in immune tumor microenvironment (TME) and blood from 87 OSCC HPV-negative patients were analyzed using a multiparameter flow cytometry assay, in a prospective case-control study. Cytokine levels in tumor supernatants and blood were determined by a cytometric bead array (CBA) assay.
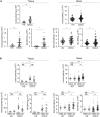
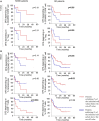
Results: Normal gingiva and blood from healthy donors (HD) were used as controls. A significant increase of granulocytes (p<0.05 for blood), of monocytes-macrophages (p<0.01 for blood) and of CD4+ T cells expressing CD45RO and CCR6 (p<0.001 for blood; p<0.0001 for TME) as well as higher levels of IL-6 (p<0.01 for sera, p<0.05 for tumor supernatant) were observed in SD patients as compared to NSND OSCC patients and HD. High percentages of CD4+ T cells expressing CD45RO and CCR6 cells in tumor tissue (p=0.05) and blood (p=0.05) of SD OSCC patients were also associated with a poorer prognosis while a high percentage of regulatory T cells (Treg) in tumor tissue was associated with a more favorable prognostic factor (p=0.05). Also, a higher percentage of blood CD8+ T lymphocytes among CD45+ cells in NSND patients was associated with a better disease-free survival (p=0.004).
Conclusion: Granulocytes, monocytes-macrophages, and CD4+ T cells expressing CD45RO and CCR6 in blood and TME as well as serum IL-6 can therefore distinguish OSCC SD and NSND patients. Quantifying the proportion of CD4+ T cells expressing CD45RO and CCR6 and of Treg in SD patients and CD8+ T cells in NSND patients could help defining the prognostic of OSCC patients.
Keywords: non-drinker; non-smoker; oral cancer; oral squamous cell carcinoma; prognostic biomarker; tumor microenvironment.
Copyright © 2023 Rochefort, Karagiannidis, Baillou, Belin, Guillot-Delost, Macedo, Le Moignic, Mateo, Soussan, Brocheriou, Teillaud, Dieu-Nosjean, Bertolus, Lemoine and Lescaille.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Satgunaseelan L, Allanson BM, Asher R, Reddy R, Low HTH, Veness M, et al. The incidence of squamous cell carcinoma of the oral tongue is rising in young non-smoking women: An international multi-institutional analysis. Oral Oncol (2020) 110:104875. doi: 10.1016/j.oraloncology.2020.104875 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

