Panax ginseng C.A. meyer alleviates benign prostatic hyperplasia while preventing finasteride-induced side effects
- PMID: 36713838
- PMCID: PMC9877295
- DOI: 10.3389/fphar.2023.1039622
Panax ginseng C.A. meyer alleviates benign prostatic hyperplasia while preventing finasteride-induced side effects
Abstract
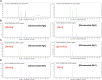
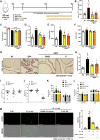
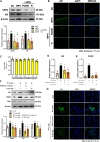
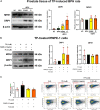
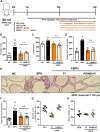
Panax ginseng C.A. Meyer, a widely used traditional medicine in East Asia, shows many beneficial effects on immune function, male erectile dysfunction, cancer, excessive oxidants, and aging issues. However, its effect on benign prostatic hyperplasia (BPH) and its potential in the treatment of side effects related to finasteride (Fi), an FDA-approved drug for BPH, are less known. This study aimed to verify the therapeutic effects of a water extract of P. ginseng (PGWE) on BPH in testosterone propionate (TP)-induced BPH rats and TP-treated RWPE-1 human epithelial cells, and the inhibitory potential on the Fi-induced side effects is also explored. In the TP-induced BPH rat model, PGWE alleviated the pathological markers of BPH such as weight and epithelial thickness of the prostate, and the serum level of dihydrotestosterone. PGWE downregulated androgen-related BPH factors such as 5α-reductase 2 and androgen receptor. PGWE also showed prostatic cell apoptosis accompanied by increased expression of Bax and decreased expression of Bcl-xL and cleaved-caspase 3, respectively, in addition to increasing mitochondrial dynamics in both in vivo and in vitro BPH models. Notably, reduced sperm count, one of the serious side effects of Fi, in the epididymis of BPH rats was recovered with PGWE treatment, suggesting less toxicity to sperm development by PGWE. PGWE also protected against Fi-induced sperm loss when PGWE was administered in combination with Fi without compromising the therapeutic effects of Fi on BPH. Based on these findings, we propose that PGWE could be an alternative therapeutic agent for BPH.
Keywords: 5α-reductase 2; androgen receptor; apoptosis; dihydrotestosterone; sperm loss.
Copyright © 2023 Park, Park, Song, Jung, Kim, Choi, Kim, Park, Kwak, Ahn, Lee and Um.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Cather J., Lane D., Heaphy M., Nelson B. (1999). Finasteride: An update and review. Cutis 64, 167–172. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

