Electrolyte imbalances and dehydration play a key role in Batrachochytrium salamandrivorans chytridiomycosis
- PMID: 36713878
- PMCID: PMC9880075
- DOI: 10.3389/fvets.2022.1055153
Electrolyte imbalances and dehydration play a key role in Batrachochytrium salamandrivorans chytridiomycosis
Erratum in
-
Corrigendum: Electrolyte imbalances and dehydration play a key role in Batrachochytrium salamandrivorans chytridiomycosis.Front Vet Sci. 2023 Mar 7;10:1164389. doi: 10.3389/fvets.2023.1164389. eCollection 2023. Front Vet Sci. 2023. PMID: 36960144 Free PMC article.
Abstract
Introduction: One of the most important emerging infectious diseases of amphibians is caused by the fungal pathogen Batrachochytrium salamandrivorans (Bsal). Bsal was recently discovered and is of global concern due to its potential to cause high mortality in amphibians, especially salamander species. To date, little has been reported on the pathophysiological effects of Bsal; however, studies of a similar fungus, B. dendrobatidis (Bd), have shown that electrolyte losses and immunosuppression likely play a key role in morbidity and mortality associated with this disease. The goal of this study was to investigate pathophysiological effects and immune responses associated with Bsal chytridiomycosis using 49 rough-skinned newts (Taricha granulosa) as the model species.
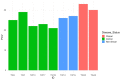
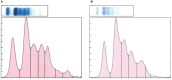
Methods: Taricha granulosa were exposed to a 1 × 107 per 10 mL dose of Bsal zoospores and allowed to reach various stages of disease progression before being humanely euthanized. At the time of euthanasia, blood was collected for biochemical and hematological analyses as well as protein electrophoresis. Ten standardized body sections were histologically examined, and Bsal-induced skin lesions were counted and graded on a scale of 1-5 based on severity.
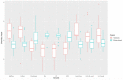
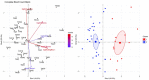
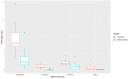
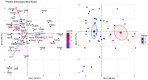
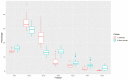
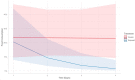
Results: Results indicated that electrolyte imbalances and dehydration induced by damage to the epidermis likely play a major role in the pathogenesis of Bsal chytridiomycosis in this species. Additionally, Bsal-infected, clinically diseased T. granulosa exhibited a systemic inflammatory response identified through alterations in complete blood counts and protein electrophoretograms.
Discussion: Overall, these results provide foundational information on the pathogenesis of this disease and highlight the differences and similarities between Bsal and Bd chytridiomycosis.
Keywords: amphibian; chytrid; clinical pathology; disease; histopathology; newt.
Copyright © 2023 Sheley, Gray, Wilber, Cray, Carter and Miller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Lotters S, Veith M, Wagner N, Martel A, Pasmans F. Bsal-driven salamander mortality pre-dates the european index outbreak. Salamandra. (2020) 56:239–42.
LinkOut - more resources
Full Text Sources

