Stromal cells support the survival of human primary chronic lymphocytic leukemia (CLL) cells through Lyn-driven extracellular vesicles
- PMID: 36714146
- PMCID: PMC9880074
- DOI: 10.3389/fmed.2022.1059028
Stromal cells support the survival of human primary chronic lymphocytic leukemia (CLL) cells through Lyn-driven extracellular vesicles
Abstract
Introduction: In chronic lymphocytic leukemia (CLL), the tumor cells receive survival support from stromal cells through direct cell contact, soluble factors and extracellular vesicles (EVs). The protein tyrosine kinase Lyn is aberrantly expressed in the malignant and stromal cells in CLL tissue. We studied the role of Lyn in the EV-based communication and tumor support.
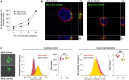
Methods: We compared the Lyn-dependent EV release, uptake and functionality using Lyn-proficient (wild-type) and -deficient stromal cells and primary CLL cells.
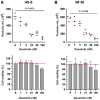
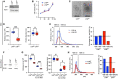
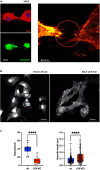
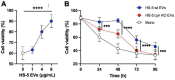
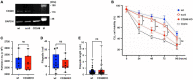
Results: Lyn-proficient cells caused a significantly higher EV release and EV uptake as compared to Lyn-deficient cells and also conferred stronger support of primary CLL cells. Proteomic comparison of the EVs from Lyn-proficient and -deficient stromal cells revealed 70 significantly differentially expressed proteins. Gene ontology studies categorized many of which to organization of the extracellular matrix, such as collagen, fibronectin, fibrillin, Lysyl oxidase like 2, integrins and endosialin (CD248). In terms of function, a knockdown of CD248 in Lyn+ HS-5 cells resulted in a diminished B-CLL cell feeding capacity compared to wildtype or scrambled control cells. CD248 is a marker of certain tumors and cancer-associated fibroblast (CAF) and crosslinks fibronectin and collagen in a membrane-associated context.
Conclusion: Our data provide preclinical evidence that the tyrosine kinase Lyn crucially influences the EV-based communication between stromal and primary B-CLL cells by raising EV release and altering the concentration of functional molecules of the extracellular matrix.
Keywords: CD248; Lyn kinase; chronic lymphocytic leukemia; extracellular matrix; extracellular vesicle; filopodia.
Copyright © 2023 de Oliveira, vom Stein, Rebollido-Rios, Lobastova, Lettau, Janssen, Wagle, Nguyen, Hallek and Hansen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Crompot E, Van Damme M, Pieters K, Vermeersch M, Perez-Morga D, Mineur P, et al. Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications. Haematologica. (2017) 102:1594–604. 10.3324/haematol.2016.163337 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

