Neural suppression in odor recognition memory
- PMID: 36715106
- PMCID: PMC9940621
- DOI: 10.1093/chemse/bjad001
Neural suppression in odor recognition memory
Abstract
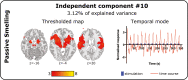
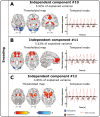
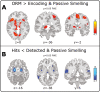
Little is known about the neural basis of lower- and higher-order olfactory functions such as odor memory, compared with other sensory systems. The aim of this study was to explore neural networks and correlates associated with 3 functions: passive smelling (PS), odor encoding (OE), and in particular odor recognition memory (ORM). Twenty-six healthy participants were examined using functional magnetic resonance imaging conducted across 3 sessions, one for each function. Independent component analysis revealed a difference between sessions where a distinct ORM component incorporating hippocampus and posterior cingulate showed delayed triggering dissociated from odor stimulation and recognition. By contrasting Hit for ORM (target odors correctly recognized as old) and a combination of PS and detected odors from OE, we found significantly lower activations in amygdala, piriform cortex, insula, thalamus, and the inferior parietal lobule. Region of interest analysis including anterior insula, posterior cingulate gyrus, dentate gyrus, left middle frontal gyrus, amygdala, and piriform cortex demonstrated that Hit were associated with lower activations compared with other memory responses. In summary, our findings suggest that successful recognition of familiar odors (odor familiarity) is associated with neural suppression in the abovementioned regions of interest. Additionally, network including the hippocampus and posterior cingulate is engaged in a postrecognition process. This process may be related to incidental encoding of less familiar and more novel odors (odor novelty) and should be subject for future research.
Keywords: episodic memory; familiarity; functional magnetic resonance imaging (fMRI); independent component analysis (ICA); olfaction; region of interest analysis (ROI).
© The Author(s) 2023. Published by Oxford University Press.
Figures


References
-
- Arshamian A, Iannilli E, Gerber JC, Willander J, Persson J, Seo HS, Hummel T, Larsson M.. The functional neuroanatomy of odor evoked autobiographical memories cued by odors and words. Neuropsychologia. 2013;51(1):123–131. - PubMed
-
- Beckmann CF, Smith SM.. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. - PubMed
-
- Beckmann CF, Smith SM.. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25(1):294–311. - PubMed
-
- Bird CM. The role of the hippocampus in recognition memory. Cortex. 2017;93:155–165. - PubMed
-
- Boesveldt S, de Muinck Keizer RJ, Wolters E, Berendse HW.. Odor recognition memory is not independently impaired in Parkinson’s disease. J Neural Transm. 2009;116(5): 575–578. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

