Genetically engineered mouse models for hereditary cancer syndromes
- PMID: 36715493
- PMCID: PMC10154891
- DOI: 10.1111/cas.15737
Genetically engineered mouse models for hereditary cancer syndromes
Abstract
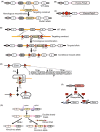
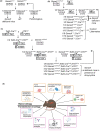
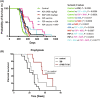
Advances in molecular diagnostics have led to improved diagnosis and molecular understanding of hereditary cancers in the clinic. Improving the management, treatment, and potential prevention of cancers in carriers of predisposing mutations requires preclinical experimental models that reflect the key pathogenic features of the specific syndrome associated with the mutations. Numerous genetically engineered mouse (GEM) models of hereditary cancer have been developed. In this review, we describe the models of Lynch syndrome and hereditary breast and ovarian cancer syndrome, the two most common hereditary cancer predisposition syndromes. We focus on Lynch syndrome models as illustrative of the potential for using mouse models to devise improved approaches to prevention of cancer in a high-risk population. GEM models are an invaluable tool for hereditary cancer models. Here, we review GEM models for some hereditary cancers and their potential use in cancer prevention studies.
Keywords: cancer genetics; disease model; genetically engineered mice; hereditary cancers; mouse models.
Published 2023. This article is a U.S. Government work and is in the public domain in the USA. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have declared that no conflict of interest exists. Dr Robert H. Shoemaker is member of the editorial board.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

