Differential chondrogenic differentiation between iPSC derived from healthy and OA cartilage is associated with changes in epigenetic regulation and metabolic transcriptomic signatures
- PMID: 36715686
- PMCID: PMC9886280
- DOI: 10.7554/eLife.83138
Differential chondrogenic differentiation between iPSC derived from healthy and OA cartilage is associated with changes in epigenetic regulation and metabolic transcriptomic signatures
Abstract
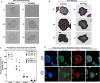
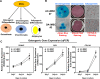
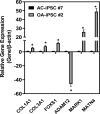
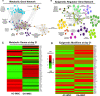
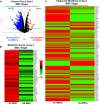
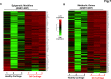
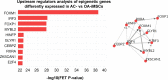
Induced pluripotent stem cells (iPSCs) are potential cell sources for regenerative medicine. The iPSCs exhibit a preference for lineage differentiation to the donor cell type indicating the existence of memory of origin. Although the intrinsic effect of the donor cell type on differentiation of iPSCs is well recognized, whether disease-specific factors of donor cells influence the differentiation capacity of iPSC remains unknown. Using viral based reprogramming, we demonstrated the generation of iPSCs from chondrocytes isolated from healthy (AC-iPSCs) and osteoarthritis cartilage (OA-iPSCs). These reprogrammed cells acquired markers of pluripotency and differentiated into uncommitted mesenchymal-like progenitors. Interestingly, AC-iPSCs exhibited enhanced chondrogenic potential as compared OA-iPSCs and showed increased expression of chondrogenic genes. Pan-transcriptome analysis showed that chondrocytes derived from AC-iPSCs were enriched in molecular pathways related to energy metabolism and epigenetic regulation, together with distinct expression signature that distinguishes them from OA-iPSCs. Our molecular tracing data demonstrated that dysregulation of epigenetic and metabolic factors seen in OA chondrocytes relative to healthy chondrocytes persisted following iPSC reprogramming and differentiation toward mesenchymal progenitors. Our results suggest that the epigenetic and metabolic memory of disease may predispose OA-iPSCs for their reduced chondrogenic differentiation and thus regulation at epigenetic and metabolic level may be an effective strategy for controlling the chondrogenic potential of iPSCs.
Keywords: cell biology; chondrocytes; human; mouse; regenerative medicine; stem cells; transcriptomics.
Conflict of interest statement
NK, MD, SC, PP, PB, LM, RG, HD No competing interests declared
Figures




Similar articles
-
Footprint-free human induced pluripotent stem cells from articular cartilage with redifferentiation capacity: a first step toward a clinical-grade cell source.Stem Cells Transl Med. 2014 Apr;3(4):433-47. doi: 10.5966/sctm.2013-0138. Epub 2014 Mar 6. Stem Cells Transl Med. 2014. PMID: 24604283 Free PMC article.
-
Differentiation of Human Induced Pluripotent Stem Cells (iPSCs)-derived Mesenchymal Progenitors into Chondrocytes.Bio Protoc. 2023 Nov 5;13(21):e4874. doi: 10.21769/BioProtoc.4874. eCollection 2023 Nov 5. Bio Protoc. 2023. PMID: 37969761 Free PMC article.
-
Alterations of Glycosphingolipid Glycans and Chondrogenic Markers during Differentiation of Human Induced Pluripotent Stem Cells into Chondrocytes.Biomolecules. 2020 Dec 1;10(12):1622. doi: 10.3390/biom10121622. Biomolecules. 2020. PMID: 33271874 Free PMC article.
-
The Potency of Induced Pluripotent Stem Cells in Cartilage Regeneration and Osteoarthritis Treatment.Adv Exp Med Biol. 2018;1079:55-68. doi: 10.1007/5584_2017_141. Adv Exp Med Biol. 2018. PMID: 29270885 Review.
-
Paracrine Interactions Involved in Human Induced Pluripotent Stem Cells Differentiation into Chondrocytes.Curr Stem Cell Res Ther. 2020;15(3):233-242. doi: 10.2174/1574888X15666191224122058. Curr Stem Cell Res Ther. 2020. PMID: 31889496 Review.
Cited by
-
Integration of Transcriptome and MicroRNA Profile Analysis of iMSCs Defines Their Rejuvenated State and Conveys Them into a Novel Resource for Cell Therapy in Osteoarthritis.Cells. 2023 Jun 30;12(13):1756. doi: 10.3390/cells12131756. Cells. 2023. PMID: 37443790 Free PMC article.
-
Mesenchymal stem cells for osteoarthritis: Recent advances in related cell therapy.Bioeng Transl Med. 2024 Aug 5;10(1):e10701. doi: 10.1002/btm2.10701. eCollection 2025 Jan. Bioeng Transl Med. 2024. PMID: 39801757 Free PMC article. Review.
-
Efficient Differentiation of Human Induced Pluripotent Stem Cell (hiPSC)-Derived Mesenchymal Progenitors Into Adipocytes and Osteoblasts.Bio Protoc. 2023 Nov 20;13(22):e4885. doi: 10.21769/BioProtoc.4885. eCollection 2023 Nov 20. Bio Protoc. 2023. PMID: 38023794 Free PMC article.
-
Classes of Stem Cells: From Biology to Engineering.Regen Eng Transl Med. 2024 Sep;10(3):309-322. doi: 10.1007/s40883-023-00317-x. Epub 2023 Sep 18. Regen Eng Transl Med. 2024. PMID: 39387056 Free PMC article.
-
Enhanced osteogenic potential of iPSC-derived mesenchymal progenitor cells following genome editing of GWAS variants in the RUNX1 gene.Bone Res. 2024 Dec 6;12(1):70. doi: 10.1038/s41413-024-00369-x. Bone Res. 2024. PMID: 39643619 Free PMC article.
References
-
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. ClueGO: a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. - DOI - PMC - PubMed
-
- Choompoo N, Bartley OJM, Precious SV, Vinh N-N, Schnell C, Garcia A, Roberton VH, Williams NM, Kemp PJ, Kelly CM, Rosser AE. Induced pluripotent stem cells derived from the developing striatum as a potential donor source for cell replacement therapy for Huntington disease. Cytotherapy. 2021;23:111–118. doi: 10.1016/j.jcyt.2020.06.001. - DOI - PMC - PubMed
-
- Diaz-Hernandez ME, Khan NM, Trochez CM, Yoon T, Maye P, Presciutti SM, Gibson G, Drissi H. Derivation of notochordal cells from human embryonic stem cells reveals unique regulatory networks by single cell-transcriptomics. Journal of Cellular Physiology. 2020;235:5241–5255. doi: 10.1002/jcp.29411. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

