A Mouse Model to Test Novel Therapeutics for Parkinson's Disease: an Update on the Thy1-aSyn ("line 61") Mice
- PMID: 36715870
- PMCID: PMC10119371
- DOI: 10.1007/s13311-022-01338-0
A Mouse Model to Test Novel Therapeutics for Parkinson's Disease: an Update on the Thy1-aSyn ("line 61") Mice
Abstract
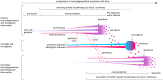
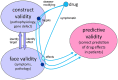
Development of neuroprotective therapeutics for Parkinson's disease (PD) is facing a lack of translation from pre-clinical to clinical trials. One strategy for improvement is to increase predictive validity of pre-clinical studies by using extensively characterized animal models with a comprehensive set of validated pharmacodynamic readouts. Mice over-expressing full-length, human, wild-type alpha-synuclein under the Thy-1 promoter (Thy1-aSyn line 61) reproduce key features of sporadic PD, such as progressive loss of striatal dopamine, alpha-synuclein pathology, deficits in motor and non-motor functions, and elevation of inflammatory markers. Extensive work with this model by multiple laboratories over the past decade further increased confidence in its robustness and validity, especially for analyzing pathomechanisms of alpha-synuclein pathology and down-stream pathways, and for pre-clinical drug testing. Interestingly, while postnatal transgene expression is widespread in central and peripheral neurons, the extent and progression of down-stream pathology differs between brain regions, thereby replicating the characteristic selective vulnerability of neurodegenerative diseases. In-depth characterization of these readouts in conjunction with behavioral deficits has led to more informative endpoints for pre-clinical trials. Each drug tested in Thy1-aSyn line 61 enhances knowledge on how molecular targets, pathology, and functional behavioral readouts are interconnected, thereby further optimizing the platform towards predictive validity for clinical trials. Here, we present the current state of the art using Thy1-aSyn line 61 for drug target discovery, validation, and pre-clinical testing.
Keywords: Alpha-synuclein; Neuroprotection; Parkinson’s disease; Progressive; Therapy.
© 2023. The Author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

