Assessment of Quality of Life Among Patients With Recurrent Clostridioides difficile Infection Treated with Investigational Oral Microbiome Therapeutic SER-109: Secondary Analysis of a Randomized Clinical Trial
- PMID: 36716031
- PMCID: PMC9887497
- DOI: 10.1001/jamanetworkopen.2022.53570
Assessment of Quality of Life Among Patients With Recurrent Clostridioides difficile Infection Treated with Investigational Oral Microbiome Therapeutic SER-109: Secondary Analysis of a Randomized Clinical Trial
Erratum in
-
Error in Visual Abstract.JAMA Netw Open. 2023 Feb 1;6(2):e234174. doi: 10.1001/jamanetworkopen.2023.4174. JAMA Netw Open. 2023. PMID: 36826823 Free PMC article. No abstract available.
Abstract
Importance: Recurrent Clostridioides difficile infection (CDI) is a debilitating disease leading to poor health-related quality of life (HRQOL), loss of productivity, anxiety, and depression. The potential association of treatment with HRQOL has not been well evaluated.
Objectives: To explore the association of SER-109 compared with placebo on HRQOL in patients with recurrent CDI up to week 8.
Design, setting, and participants: This study was a secondary analysis of a randomized, double-blind, placebo-controlled trial that took place at 56 sites in the US and Canada from July 2017 to April 2020 and included 182 patients randomized to SER-109 or placebo groups.
Interventions: SER-109 or placebo (4 capsules once daily for 3 days) following antibiotics for CDI.
Main outcomes and measures: Exploratory analysis of HRQOL using the disease specific Clostridioides difficile Quality of Life Survey (Cdiff32) assessed at baseline, week 1, and week 8.
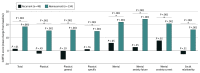
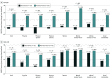
Results: In this study, 182 patients (109 [59.9%] female; mean age, 65.5 [16.5] years) were randomized to SER-109 (89 [48.9%]) or placebo (93 [51.1%]) groups and were included in the primary and exploratory analyses. Baseline Cdiff32 scores were similar between patients in the SER-109 and placebo groups (52.0 [18.3] vs 52.8 [18.7], respectively). The proportion of patients with overall improvement from baseline in the Cdiff32 total score was higher in the SER-109 arm than placebo at week 1 (49.4% vs 26.9%; P = .012) and week 8 (66.3% vs 48.4%; P = .001).Greater improvements in total and physical domain and subdomain scores were observed in patients in the SER-109 group compared with placebo as early as week 1, with continued improvements observed at week 8. Among patients in the placebo group, improvements in HRQOL were primarily observed in patients with nonrecurrent CDI while patients in the SER-109 group reported improvements in HRQOL, regardless of clinical outcome.
Conclusions and relevance: In this secondary analysis of a phase 3 clinical trial, SER-109, an investigational microbiome therapeutic was associated with rapid and steady improvement in HRQOL compared with placebo through 8 weeks, an important patient-reported outcome.
Trial registration: ClinicalTrials.gov Identifier: NCT03183128.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

