Shape-Dependent CO2 Hydrogenation to Methanol over Cu2O Nanocubes Supported on ZnO
- PMID: 36716273
- PMCID: PMC9912329
- DOI: 10.1021/jacs.2c11540
Shape-Dependent CO2 Hydrogenation to Methanol over Cu2O Nanocubes Supported on ZnO
Abstract
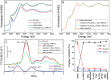
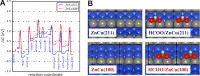
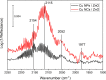
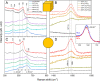
The hydrogenation of CO2 to methanol over Cu/ZnO-based catalysts is highly sensitive to the surface composition and catalyst structure. Thus, its optimization requires a deep understanding of the influence of the pre-catalyst structure on its evolution under realistic reaction conditions, including the formation and stabilization of the most active sites. Here, the role of the pre-catalyst shape (cubic vs spherical) in the activity and selectivity of ZnO-supported Cu nanoparticles was investigated during methanol synthesis. A combination of ex situ, in situ, and operando microscopy, spectroscopy, and diffraction methods revealed drastic changes in the morphology and composition of the shaped pre-catalysts under reaction conditions. In particular, the rounding of the cubes and partial loss of the (100) facets were observed, although such motifs remained in smaller domains. Nonetheless, the initial pre-catalyst structure was found to strongly affect its subsequent transformation in the course of the CO2 hydrogenation reaction and activity/selectivity trends. In particular, the cubic Cu particles displayed an increased activity for methanol production, although at the cost of a slightly reduced selectivity when compared to similarly sized spherical particles. These findings were rationalized with the help of density functional theory calculations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Schimpf S.; Muhler M.Methanol Catalysts. In Synthesis of Solid Catalysts; De Jong K. P., Ed.; Wiley-VCH Verlag, 2009, 329–351.
-
- Sehested J. Industrial and Scientific Directions of Methanol Catalyst Development. J. Catal. 2019, 371, 368–375. 10.1016/j.jcat.2019.02.002. - DOI
-
- Roy S.; Cherevotan A.; Peter S. C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Technological Challenges. ACS Energy Lett. 2018, 3, 1938–1966. 10.1021/acsenergylett.8b00740. - DOI
-
- Li M. M. J.; Tsang S. C. E. Bimetallic Catalysts for Green Methanol Production via CO2 and Renewable Hydrogen: A Mini-Review and Prospects. Catal. Sci. Technol. 2018, 8, 3450–3464. 10.1039/C8CY00304A. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

