Curvature sensing as an emergent property of multiscale assembly of septins
- PMID: 36716363
- PMCID: PMC9963131
- DOI: 10.1073/pnas.2208253120
Curvature sensing as an emergent property of multiscale assembly of septins
Abstract
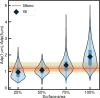
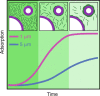
The ability of cells to sense and communicate their shape is central to many of their functions. Much is known about how cells generate complex shapes, yet how they sense and respond to geometric cues remains poorly understood. Septins are GTP-binding proteins that localize to sites of micrometer-scale membrane curvature. Assembly of septins is a multistep and multiscale process, but it is unknown how these discrete steps lead to curvature sensing. Here, we experimentally examine the time-dependent binding of septins at different curvatures and septin bulk concentrations. These experiments unexpectedly indicated that septins' curvature preference is not absolute but rather is sensitive to the combinations of membrane curvatures present in a reaction, suggesting that there is competition between different curvatures for septin binding. To understand the physical underpinning of this result, we developed a kinetic model that connects septins' self-assembly and curvature-sensing properties. Our experimental and modeling results are consistent with curvature-sensitive assembly being driven by cooperative associations of septin oligomers in solution with the bound septins. When combined, the work indicates that septin curvature sensing is an emergent property of the multistep, multiscale assembly of membrane-bound septins. As a result, curvature preference is not absolute and can be modulated by changing the physicochemical and geometric parameters involved in septin assembly, including bulk concentration, and the available membrane curvatures. While much geometry-sensitive assembly in biology is thought to be guided by intrinsic material properties of molecules, this is an important example of how curvature sensing can arise from multiscale assembly of polymers.
Keywords: curvature sensing; kinetic modeling; multiscale assembly; septin.
Conflict of interest statement
The authors declare no competing interest.
Figures
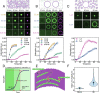
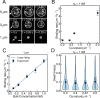
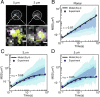
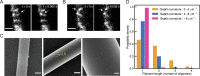

References
-
- Peter B. J., et al. , BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 303, 495–499 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

